Why Do Different Chemicals Emit Different Colors Of Light
Kalali
Mar 14, 2025 · 5 min read
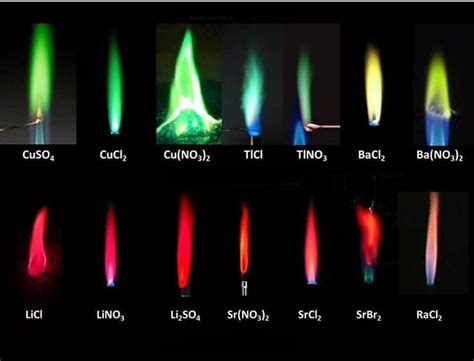
Table of Contents
Why Do Different Chemicals Emit Different Colors of Light?
The vibrant hues of fireworks, the soothing glow of neon signs, and the intense brilliance of a flame – these are all examples of light emission from chemicals. But why do different chemicals produce different colors? The answer lies in the fascinating world of atomic structure and the quantum nature of light. Understanding this phenomenon requires delving into the intricacies of electron energy levels, photon emission, and the relationship between wavelength and color.
The Atomic Basis of Light Emission
At the heart of this phenomenon is the atom, the fundamental building block of matter. Each atom consists of a nucleus containing protons and neutrons, surrounded by orbiting electrons. These electrons don't orbit randomly; they exist in specific energy levels or shells. Each shell can hold a limited number of electrons. The farther an electron is from the nucleus, the higher its energy level.
Electrons and Energy Levels: Electrons are naturally drawn to lower energy levels, closer to the nucleus. When an electron absorbs energy—from heat, electricity, or a chemical reaction—it can "jump" to a higher energy level. This is an unstable state, however. The electron wants to return to its original, lower energy level.
The Role of Photons
This return to a lower energy level is where the light emission happens. When an electron drops from a higher energy level to a lower one, it releases the excess energy as a photon, a fundamental particle of light. The energy of the photon is directly related to the energy difference between the two energy levels.
Energy and Wavelength: The energy of a photon determines its wavelength, and the wavelength directly dictates the color of the light we see. Higher-energy photons have shorter wavelengths and appear as violet or blue light. Lower-energy photons have longer wavelengths and appear as red or orange light. This relationship is described by the equation: E = hc/λ, where E is the energy, h is Planck's constant, c is the speed of light, and λ is the wavelength.
Different chemicals have unique atomic structures, meaning they have different arrangements of electrons in different energy levels. When these chemicals are energized, their electrons jump to higher energy levels and subsequently fall back, emitting photons with specific energies and therefore, specific wavelengths and colors.
Why Different Elements Produce Different Colors
The uniqueness of the atomic structure of an element is the primary reason why each element produces a characteristic color when excited. For example:
-
Sodium (Na): Sodium atoms have a particular electron configuration. When energized, their electrons jump to higher energy levels and then fall back, releasing photons with energy corresponding to the yellow-orange region of the electromagnetic spectrum. This is why sodium streetlights appear yellow-orange.
-
Copper (Cu): Copper atoms have a different electron configuration than sodium. When excited, they emit photons in the blue-green region of the spectrum, resulting in the characteristic blue-green color observed in some fireworks.
-
Lithium (Li): Lithium's electron configuration leads to the emission of photons in the red region of the spectrum. Thus, lithium compounds are frequently used in fireworks to create red colors.
The Influence of Electron Transitions
The specific color emitted isn't determined by just the element itself but also by the specific electron transitions occurring within the atom. An atom can have many possible energy levels, and electrons can jump between various combinations of these levels. Each transition corresponds to a different energy difference and hence, a different photon energy and wavelength. This explains why a single element can sometimes produce multiple colors under different excitation conditions or in the presence of other chemicals.
The Role of Chemical Compounds
The situation becomes even more complex when considering chemical compounds rather than individual elements. In a compound, the atoms are bound together, and the energy levels are influenced by the interactions between the atoms. This leads to a wider range of possible electron transitions and therefore, a wider variety of emitted colors.
For example, the color of a flame can change dramatically depending on the chemical compounds present. Burning magnesium produces a bright white light, while burning copper compounds often result in a bluish-green flame. The presence of different elements within the compound modifies the electronic structure and the energy transitions, leading to altered light emission.
Factors Affecting Color Intensity
Beyond the fundamental atomic structure and energy levels, several other factors contribute to the intensity and clarity of the emitted color:
-
Temperature: Higher temperatures generally lead to more energetic electron transitions and a greater intensity of emitted light.
-
Concentration: The concentration of the emitting chemical also influences the intensity. Higher concentrations usually mean more atoms are available to emit light, resulting in brighter colors.
-
Presence of other elements: The presence of other elements can influence the electron energy levels within a given chemical, altering the color emitted. This is sometimes used in fireworks to create more nuanced and complex color combinations.
-
Flame composition: The chemical composition of the flame itself can affect the light emission process. For instance, the presence of oxygen can significantly impact the intensity and color of the light emitted from a burning substance.
Spectroscopic Analysis and Identification
The relationship between chemical composition and emitted light is so reliable that scientists utilize it in spectroscopy. Spectroscopy is a technique that analyzes the emitted light from a substance to determine its composition. Each element and compound has a unique "fingerprint" – a distinct pattern of wavelengths and intensities in its emitted light – allowing for its identification. This technique is used across numerous scientific fields, from astronomy to forensic science.
Conclusion: A Symphony of Colors from the Quantum World
The vibrant colors produced by different chemicals are not arbitrary; they arise from the precise interactions between electrons and the quantum nature of light. The unique atomic structure of each element and the specific electron transitions that occur when the atoms are energized dictate the wavelength and intensity of the emitted photons. This simple, yet fundamental, principle explains the captivating array of colors we see in fireworks, neon signs, flames, and numerous other natural and man-made phenomena. The intricate interplay between energy levels, electron transitions, and photon emission paints a colorful picture of the quantum world, highlighting the elegance and predictability of its rules. This understanding has profound implications for scientific advancements and the appreciation of the beautiful world around us.
Latest Posts
Latest Posts
-
What Is Double Of 1 4 Cup
Jul 10, 2025
-
Prepare Me A Body And I Will Redeem Man
Jul 10, 2025
-
How Many Inches Is A Meter Stick
Jul 10, 2025
-
Soundtrack To Step Up 2 The Streets
Jul 10, 2025
-
Keebler Club And Cheddar Crackers Expiration Date
Jul 10, 2025
Related Post
Thank you for visiting our website which covers about Why Do Different Chemicals Emit Different Colors Of Light . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.