Why Do Different Elements Emit Different Colors
Kalali
Mar 17, 2025 · 5 min read
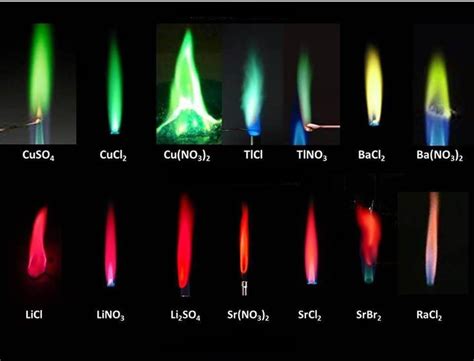
Table of Contents
Why Do Different Elements Emit Different Colors?
The vibrant hues of fireworks, the soothing glow of neon signs, and the spectral lines analyzed in astronomy all stem from a single fundamental principle: the unique atomic structure of each element dictates the specific colors it emits. This phenomenon, known as atomic emission, is a cornerstone of spectroscopy and has far-reaching implications in various fields, from materials science to astrophysics. Understanding why different elements produce distinct colors requires delving into the quantum world of electrons and energy levels.
The Quantum Leap: Electron Energy Levels
Atoms are composed of a nucleus containing protons and neutrons, surrounded by orbiting electrons. Unlike classical physics, where electrons could theoretically orbit at any distance from the nucleus, quantum mechanics dictates that electrons can only occupy specific energy levels. These levels are quantized, meaning they exist as discrete, distinct values, not a continuous spectrum. Think of it like a staircase – you can only stand on specific steps, not in between.
Each element possesses a unique arrangement of these energy levels, determined by the number of protons (atomic number) in its nucleus and the resulting electromagnetic interactions with the electrons. These energy levels are not randomly distributed; they are organized into shells and subshells, each capable of holding a specific number of electrons. The further an electron is from the nucleus, the higher its energy level.
Ground State and Excited States
In its most stable state, an atom is said to be in its ground state. All its electrons occupy the lowest available energy levels. However, atoms can absorb energy from external sources, such as heat, electricity, or light. This absorbed energy can boost an electron to a higher energy level, a process called excitation. The atom is now in an excited state.
This excited state is inherently unstable. The electron will quickly transition back to a lower energy level, releasing the excess energy as a photon, a particle of light. The energy of this photon is directly proportional to the difference in energy between the two levels involved in the transition.
The Color Connection: Wavelength and Energy
The crucial link between atomic emission and color lies in the relationship between the energy of the photon and its wavelength (or frequency). The energy of a photon is inversely proportional to its wavelength: higher energy photons have shorter wavelengths, and lower energy photons have longer wavelengths.
The visible spectrum of light, the range of wavelengths our eyes can perceive, corresponds to a specific range of photon energies. Different wavelengths within this spectrum appear as different colors to us:
- Red: Longest wavelength, lowest energy
- Orange:
- Yellow:
- Green:
- Blue:
- Indigo:
- Violet: Shortest wavelength, highest energy
Because each element has a unique arrangement of energy levels, the energy differences between these levels are also unique. Consequently, the photons emitted during electron transitions will have specific energies and therefore specific wavelengths, resulting in the emission of characteristic colors.
Spectroscopic Fingerprints: Identifying Elements
This unique color signature of each element is the basis of atomic emission spectroscopy. By analyzing the light emitted by a heated or electrically excited sample of an element, scientists can identify its composition by observing the specific wavelengths (and hence colors) of light present. This technique is incredibly powerful and has numerous applications:
Astronomical Spectroscopy
Astronomers use spectroscopy to determine the composition of stars and nebulae. By analyzing the light emitted by celestial objects, they can identify the elements present and even measure their abundance. This provides valuable insights into the formation and evolution of stars and galaxies. The characteristic spectral lines of hydrogen, helium, and other elements are readily detectable in starlight.
Forensic Science
Emission spectroscopy plays a vital role in forensic science. It can be used to analyze trace evidence, such as paint chips or gunshot residue, to identify their chemical composition and link them to a crime scene or suspect. The precision of spectral analysis allows for highly reliable identification.
Material Science
In material science, spectroscopy is employed to study the properties of materials at the atomic level. It helps determine the purity of substances and identify impurities that may affect their performance. The technique is instrumental in quality control and research and development efforts.
Examples of Characteristic Colors:
While the exact color depends on the specific energy level transitions, some elements are associated with particular prominent colors:
-
Sodium (Na): Produces a bright, intense yellow light, a common sight in sodium-vapor street lamps. This distinctive yellow originates from the transition of electrons between the 3p and 3s energy levels.
-
Lithium (Li): Emits a characteristic crimson red light.
-
Potassium (K): Displays a lilac or violet color in flame tests.
-
Calcium (Ca): Produces a brick-red or orange-red emission.
-
Copper (Cu): Exhibits a vibrant blue-green color in flame tests.
These characteristic colors are not arbitrary; they are precisely determined by the unique energy level differences within each element's atomic structure.
Beyond the Visible Spectrum:
It's crucial to note that atomic emission doesn't just occur in the visible spectrum. Elements also emit radiation at wavelengths outside the visible range, including ultraviolet (UV) and infrared (IR). While we can't see these wavelengths with our naked eyes, specialized detectors can measure them, expanding the scope of spectroscopic analysis. This broader perspective provides even more detailed information about the elemental composition and energy levels of a sample.
Factors Influencing Emission Color Intensity:
While the specific colors emitted are inherent to the element's atomic structure, several factors can influence the intensity of the emission:
-
Temperature: Higher temperatures generally lead to more intense emission lines as more atoms are excited to higher energy levels.
-
Concentration: A higher concentration of the element results in a brighter emission.
-
Pressure: Pressure can affect the broadening and shifting of spectral lines.
-
Presence of other elements: Interactions between different elements in a sample can subtly alter the emission spectrum.
Conclusion:
The production of different colors by different elements is a direct consequence of their unique atomic structures and the quantized nature of electron energy levels. The specific wavelengths of light emitted, and thus the colors observed, are a unique fingerprint of each element. This phenomenon, extensively utilized in spectroscopy, has revolutionized many scientific fields, allowing for the precise identification and analysis of matter at the atomic level, from terrestrial samples to distant celestial objects. Understanding this fundamental principle provides a deeper appreciation for the intricate workings of the quantum world and its profound impact on our understanding of the universe.
Latest Posts
Latest Posts
-
3 Feet 6 Inches In Cm
Mar 17, 2025
-
What Is 26 Out Of 30 As A Percentage
Mar 17, 2025
-
How Many Feet Is 26 In
Mar 17, 2025
-
What Is 6 Out Of 20 As A Percentage
Mar 17, 2025
-
What Is Melting Point Of Glass
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Why Do Different Elements Emit Different Colors . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
