Why Does Fluorine Have A Higher Ionization Energy Than Iodine
Kalali
Apr 06, 2025 · 5 min read
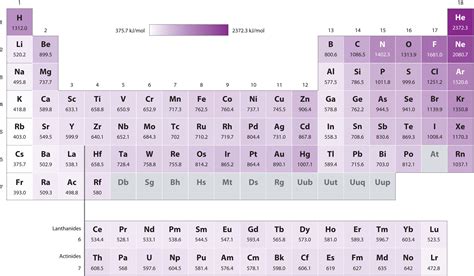
Table of Contents
Why Does Fluorine Have a Higher Ionization Energy Than Iodine? A Deep Dive into Periodic Trends
The periodic table is a treasure trove of information, organizing elements based on their atomic structure and resulting properties. One crucial property is ionization energy – the energy required to remove an electron from a gaseous atom. A seemingly simple concept, it reveals a fascinating story about the interplay of nuclear charge, atomic size, and electron shielding. This article delves into the reasons why fluorine boasts a significantly higher ionization energy than iodine, exploring the underlying principles and addressing common misconceptions.
Understanding Ionization Energy
Before we compare fluorine and iodine, let's establish a firm understanding of ionization energy. The first ionization energy (IE₁) specifically refers to the energy needed to remove the first electron from a neutral atom. Subsequent ionization energies (IE₂, IE₃, etc.) represent the energy required to remove further electrons from increasingly positively charged ions. These values systematically increase for each successive electron removal because of the increasing positive charge of the remaining ion.
The process can be represented as:
X(g) + energy → X⁺(g) + e⁻
Where:
- X(g) is a gaseous atom of element X
- X⁺(g) is a gaseous ion of element X with a +1 charge
- e⁻ is an electron
The Role of Atomic Radius and Effective Nuclear Charge
The key factors determining ionization energy are:
-
Effective Nuclear Charge (Z<sub>eff</sub>): This refers to the net positive charge experienced by an electron in an atom. It's the difference between the actual nuclear charge (number of protons) and the shielding effect of inner electrons. Electrons in inner shells repel outer electrons, reducing the attractive force from the nucleus.
-
Atomic Radius: The distance between the nucleus and the outermost electrons. A larger atomic radius means the outermost electrons are further from the nucleus and experience a weaker attractive force.
In essence, a higher effective nuclear charge and a smaller atomic radius result in a higher ionization energy. The electrons are held more tightly, requiring more energy to remove them.
Fluorine vs. Iodine: A Comparative Analysis
Now let's contrast fluorine (F) and iodine (I), both belonging to Group 17 (halogens) but occupying significantly different positions on the periodic table.
Fluorine (F)
- Atomic Number: 9
- Electronic Configuration: [He] 2s²2p⁵
- Small Atomic Radius: The outermost electrons are relatively close to the nucleus.
- High Effective Nuclear Charge: The small size and relatively few electrons result in a strong net positive charge experienced by the valence electrons. There's minimal shielding from inner electrons.
Iodine (I)
- Atomic Number: 53
- Electronic Configuration: [Kr] 5s²4d¹⁰5p⁵
- Large Atomic Radius: The outermost electrons are significantly farther from the nucleus compared to fluorine.
- Lower Effective Nuclear Charge: The numerous inner electrons effectively shield the outer electrons from the positive nuclear charge, reducing the net attractive force.
Why Fluorine's Ionization Energy is Higher
The significant difference in ionization energies between fluorine and iodine stems directly from the differences in atomic radius and effective nuclear charge:
-
Smaller Atomic Radius: Fluorine's much smaller atomic radius means its outermost electron is much closer to the positively charged nucleus. The electrostatic attraction between the nucleus and the electron is substantially stronger in fluorine.
-
Higher Effective Nuclear Charge: Despite having more protons, iodine's large number of inner electrons significantly shields the outermost electron from the nucleus. This shielding effect reduces the effective nuclear charge experienced by the outermost electron in iodine, making it easier to remove compared to fluorine.
-
Increased Electron-Electron Repulsion in Iodine: With more electrons in iodine, there's increased electron-electron repulsion amongst the valence electrons. This repulsion partially offsets the attractive force from the nucleus, making it easier to remove an electron.
Addressing Common Misconceptions
Several misconceptions often arise when discussing ionization energy trends:
-
Incorrect Assumption about Electron Shielding: Some mistakenly believe that increased electron shielding always leads to lower ionization energy. While generally true, the effect of increased shielding is often overshadowed by the larger distance of the valence electrons from the nucleus in larger atoms. Iodine, despite its higher shielding, has a much larger atomic radius, which significantly reduces the overall attractive force on the outermost electron.
-
Ignoring the Role of Atomic Radius: Focusing solely on effective nuclear charge without considering the atomic radius leads to an incomplete understanding. Both factors must be considered together for a complete picture.
-
Oversimplification of Electron-Electron Repulsion: While electron-electron repulsion plays a role, it's not the primary factor determining the vast difference between fluorine and iodine's ionization energies. The dominant factors remain atomic radius and effective nuclear charge.
Beyond the First Ionization Energy
It's crucial to remember that the discussion above primarily focuses on the first ionization energy. Subsequent ionization energies will always be higher, reflecting the increasing positive charge of the ion and the stronger attraction for the remaining electrons. This trend holds true for both fluorine and iodine.
Conclusion
In summary, fluorine exhibits a considerably higher ionization energy than iodine because of its substantially smaller atomic radius and higher effective nuclear charge. The closer proximity of the outermost electrons to the nucleus in fluorine leads to a significantly stronger electrostatic attraction, requiring more energy to remove an electron. While increased electron shielding and electron-electron repulsion in iodine play a role, the effects of atomic radius and effective nuclear charge are far more dominant in determining the difference in ionization energies between these two halogens. Understanding these fundamental principles is essential for grasping the periodic trends and the behavior of elements.
Latest Posts
Latest Posts
-
How Much Inches Is 18 Cm
Apr 07, 2025
-
Cuantos Pies Cuadrados Tiene Un Metro
Apr 07, 2025
-
What Is 90 Degrees In Celsius
Apr 07, 2025
-
How Much Is 150cm In Inches
Apr 07, 2025
-
How Many Feet In 230 Cm
Apr 07, 2025
Related Post
Thank you for visiting our website which covers about Why Does Fluorine Have A Higher Ionization Energy Than Iodine . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.