Why Does Stirring Increase The Rate Of Dissolution
Kalali
Apr 01, 2025 · 5 min read
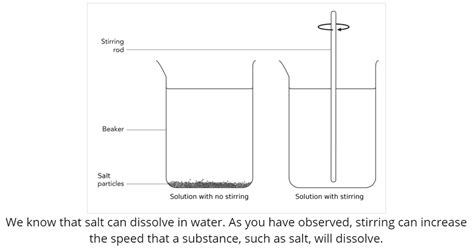
Table of Contents
Why Does Stirring Increase the Rate of Dissolution?
Dissolution, the process where a solid substance dissolves into a liquid to form a solution, is a fundamental concept in chemistry and countless everyday applications. Understanding the factors that influence the rate of dissolution is crucial in various fields, from pharmacy and chemical engineering to cooking and environmental science. One of the most readily observable factors affecting dissolution rate is stirring. But why does stirring significantly increase the rate at which a solid dissolves? This article delves deep into the scientific principles behind this phenomenon, exploring the underlying mechanisms and practical implications.
Understanding the Dissolution Process
Before we delve into the effects of stirring, let's establish a foundational understanding of the dissolution process itself. Dissolution isn't simply a substance disappearing; it's a complex interplay of several factors:
1. The Nature of the Solute and Solvent:
The intrinsic properties of both the solute (the substance dissolving) and the solvent (the liquid doing the dissolving) play a critical role. Polar solvents, like water, tend to dissolve polar solutes (like salt), while nonpolar solvents, like oil, dissolve nonpolar solutes (like fats). This is due to the principle of "like dissolves like," where similar intermolecular forces facilitate interaction and dissolution. The solubility of a substance—the maximum amount that can dissolve in a given amount of solvent at a specific temperature—is inherent to the solute-solvent pair.
2. Surface Area:
The surface area of the solute exposed to the solvent is a crucial determinant of dissolution rate. A larger surface area provides more contact points between the solute and solvent molecules, accelerating the dissolution process. This is why powdered sugar dissolves faster than a sugar cube—the powder has a drastically larger surface area.
3. Temperature:
Higher temperatures generally increase the rate of dissolution. Increased kinetic energy of both solute and solvent molecules leads to more frequent and energetic collisions, facilitating the breaking of intermolecular forces holding the solute together and enabling its incorporation into the solvent.
4. Concentration Gradient:
A concentration gradient exists between the area of high solute concentration (near the undissolved solid) and the area of lower concentration (further away in the solution). Dissolution is driven by the tendency to equalize this gradient, moving solute molecules from areas of high concentration to areas of low concentration.
The Role of Stirring in Enhancing Dissolution
Stirring directly impacts the rate of dissolution by addressing several of the factors mentioned above:
1. Increasing Surface Area:
While not directly increasing the total surface area of the solid, stirring continuously exposes fresh surfaces of the solid solute to the solvent. Without stirring, the dissolved solute molecules might accumulate around the solid, creating a layer of high concentration that hinders further dissolution (this is known as a boundary layer). Stirring disrupts this boundary layer, constantly replenishing the solvent in contact with the solute.
2. Enhancing the Concentration Gradient:
Stirring effectively reduces the concentration gradient by continuously mixing the solution. This constant mixing prevents the build-up of solute molecules near the surface of the solid, maintaining a strong driving force for further dissolution. The solution remains relatively uniform in concentration, ensuring that the solvent remains undersaturated and readily available to dissolve more solute.
3. Increasing the Rate of Collisions:
Stirring increases the frequency and energy of collisions between solvent molecules and the surface of the solute. These collisions are crucial for breaking the intermolecular forces holding the solute together and transferring solute molecules into the solvent. The more frequent and energetic the collisions, the faster the dissolution.
4. Minimizing Diffusion Limitations:
The process of dissolution involves not only the breaking of solute bonds but also the diffusion of dissolved solute molecules through the solution. Stirring minimizes the distance that dissolved molecules need to travel, accelerating this diffusion process and preventing the formation of regions of high concentration that would otherwise slow down dissolution.
Practical Implications and Examples
The impact of stirring on dissolution rate has far-reaching implications in many practical applications:
-
Pharmaceuticals: The rate at which a drug dissolves in the body significantly affects its bioavailability (the extent to which it is absorbed and becomes available to the body). Stirring or shaking a medication before administration can enhance its dissolution and, consequently, its effectiveness.
-
Chemical Engineering: In industrial processes involving dissolution, stirring is often crucial for maintaining efficient reaction rates and product quality. For example, in the production of certain chemicals, the dissolution of reactants is a crucial step, and efficient stirring ensures the process occurs at the desired rate.
-
Food Science: Dissolving sugar or salt in water for culinary purposes is faster and more efficient with stirring. The improved dissolution rate ensures even distribution of flavor and prevents clumping.
-
Environmental Science: The dissolution of pollutants in water bodies is relevant in understanding their environmental fate and transport. Water currents and mixing can act as a form of "natural stirring," influencing the rate at which pollutants dissolve and disperse.
Factors that Influence the Effectiveness of Stirring
While stirring generally increases the dissolution rate, its effectiveness can be influenced by various factors:
-
Stirring Speed: Faster stirring generally leads to faster dissolution, up to a certain point. Beyond a certain speed, the gains become marginal, and excessive stirring might even cause problems like splashing or aeration.
-
Viscosity of the Solvent: In highly viscous solvents, the benefits of stirring might be less pronounced because the mixing is hindered by the solvent's resistance to flow.
-
Size and Shape of the Solute: Very large or irregularly shaped solute particles might not fully benefit from stirring due to limitations in the exposure of fresh surfaces.
-
Presence of Other Substances: The presence of other substances in the solution (e.g., surfactants or other solutes) can influence the effectiveness of stirring by affecting the viscosity or interaction between the solute and solvent.
Conclusion: Stirring - A Catalyst for Dissolution
Stirring significantly enhances the rate of dissolution by impacting several key factors that govern the process. It disrupts boundary layers, improves concentration gradients, increases the frequency of solute-solvent collisions, and accelerates the diffusion of dissolved molecules. Understanding the mechanisms by which stirring accelerates dissolution is critical in diverse fields, from medicine to manufacturing and environmental science. While the specific effectiveness of stirring is influenced by various factors, its role as a crucial catalyst for efficient and rapid dissolution remains undeniable. Optimizing stirring techniques can enhance many processes that rely on dissolution, improving efficiency, product quality, and outcomes across various applications.
Latest Posts
Latest Posts
-
180 Out Of 240 As A Percentage
Apr 02, 2025
-
Greatest Common Multiple Of 6 And 15
Apr 02, 2025
-
How Many Ml In 5 Liters
Apr 02, 2025
-
Inventions That Use Light Reflection To Work
Apr 02, 2025
-
How Much Is 4 6 Quarts
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Why Does Stirring Increase The Rate Of Dissolution . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
