Why Is Density A Derived Unit
Kalali
Mar 17, 2025 · 6 min read
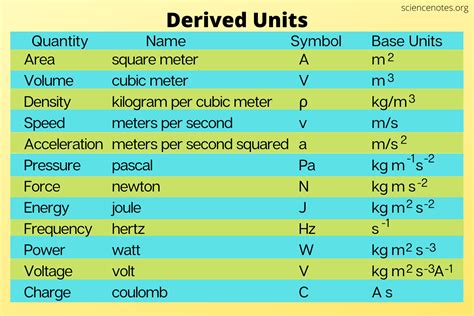
Table of Contents
Why is Density a Derived Unit? A Deep Dive into the Fundamentals of Measurement
Density, a fundamental concept in physics and chemistry, is often described as a derived unit. But what does that actually mean? Why isn't it a base unit like length, mass, or time? This article delves deep into the definition of density, explores why it's classified as a derived unit, and examines its implications in various scientific fields. We'll also touch upon related concepts like specific gravity and how they relate to the derived nature of density.
Understanding Base and Derived Units
Before understanding why density is a derived unit, we need to grasp the concept of base and derived units within the International System of Units (SI). The SI system, the most widely used system of measurement globally, defines seven base units:
- Length: meter (m)
- Mass: kilogram (kg)
- Time: second (s)
- Electric current: ampere (A)
- Thermodynamic temperature: kelvin (K)
- Amount of substance: mole (mol)
- Luminous intensity: candela (cd)
These base units are fundamental and independent of each other. They are not defined in terms of other units. All other units in the SI system are derived from these seven base units through mathematical relationships. These are called derived units.
Defining Density: Mass per Unit Volume
Density is defined as the mass per unit volume of a substance. This means it quantifies how much mass is packed into a given volume. The formula for density is:
Density (ρ) = Mass (m) / Volume (V)
This simple equation reveals the reason density is a derived unit. Both mass (kilogram, kg) and volume (cubic meter, m³) are themselves derived units (volume is derived from length – m³). Therefore, density, being a combination of these derived units, is also a derived unit. Its SI unit is kilograms per cubic meter (kg/m³).
Why Not a Base Unit? Density's Dependence on Other Properties
Density isn't considered a base unit because its value is dependent on other physical properties: mass and volume. A substance's density isn't an intrinsic, independent characteristic like its atomic number or specific heat capacity. Instead, it's a property that arises from the relationship between the mass and volume of the substance.
Imagine two identical containers, one filled with water and the other with lead. Both containers have the same volume. However, the lead container has a significantly greater mass than the water container, leading to a much higher density for lead. This demonstrates how density is intrinsically linked to the concepts of mass and volume, both of which are defined by base units.
The dependence on mass and volume makes density a property that is calculated rather than directly measured using a single fundamental instrument. This contrasts with base units, which are measured using specifically defined instruments and standards. For example, length is measured using a standard meter, while mass is measured using a calibrated balance.
Density's Importance Across Disciplines
The derived nature of density doesn't diminish its importance. Density is a critical concept in various scientific disciplines, including:
1. Physics: Fluid Mechanics and Buoyancy
Density plays a crucial role in understanding fluid mechanics, particularly in the principles of buoyancy. Archimedes' principle, a cornerstone of fluid mechanics, directly relates an object's buoyant force to the density of the fluid it's submerged in. Objects less dense than the fluid float, while those denser than the fluid sink.
2. Chemistry: Identifying Substances and Solutions
Density is a useful physical property for identifying substances. Different substances typically have distinct densities, making density a valuable tool in chemical analysis and identification. This is particularly useful for distinguishing between liquids and solids.
3. Geology: Understanding Rock Formation and Mineral Composition
Density measurements help geologists understand the composition of rocks and minerals. Different mineral compositions yield different densities, aiding in the identification and classification of rock types.
4. Engineering: Material Selection and Design
Engineers use density in material selection and design processes. The density of a material is a factor in determining structural strength, weight, and overall performance in applications ranging from aerospace to civil engineering.
5. Astronomy and Astrophysics: Stellar Structure and Planetary Formation
In astronomy, density plays a crucial role in understanding the structure of stars and planets. The density profile of a star, for instance, influences its evolutionary path and lifespan.
Specific Gravity: A Related Concept
Specific gravity is a related concept that helps to avoid the use of complicated units in everyday life, particularly when we need to determine the relative densities of liquids. It's defined as the ratio of the density of a substance to the density of a reference substance, typically water at 4°C (its maximum density). The formula is:
Specific Gravity = Density of Substance / Density of Water at 4°C
Specific gravity is a dimensionless quantity, meaning it doesn't have units. This is because the units in the numerator and denominator cancel each other out. Since it's a ratio of densities, it indirectly reflects the derived nature of density.
Practical Applications of Density Calculations
The calculation of density, given its dependence on mass and volume, is ubiquitous in various practical applications. Here are a few examples:
- Determining the volume of an irregularly shaped object: If the mass of an object is known, its density can be used to calculate its volume. This is useful for objects whose shapes are not easily measurable directly.
- Estimating the purity of a substance: Deviations in density from established values can indicate impurities in a sample.
- Determining the concentration of a solution: The density of a solution is related to its concentration, making density measurements a simple method for concentration determination.
- Calculating the mass of a substance given its volume: Knowing the density allows for the calculation of the mass of a given volume of a substance. This is useful in various industrial processes where mass needs to be accurately determined.
Conclusion: Density's Derived Status and Practical Significance
The fact that density is a derived unit – calculated from mass and volume – doesn't diminish its immense importance across numerous scientific and engineering disciplines. Its dependence on these fundamental properties reflects its nature as a relational quantity that provides vital insights into the physical characteristics of substances. Understanding the concept of base and derived units, along with the practical applications of density calculations, are crucial for anyone working with materials, fluids, or substances in any scientific or engineering field. The ease of calculating it, and the wide range of applications, make density a powerful tool for understanding the world around us. Its derived nature only underscores the elegance and interconnectedness of the fundamental concepts in physics and chemistry.
Latest Posts
Latest Posts
-
Is 15 A Prime Or Composite Number
Aug 20, 2025
-
I Ll Miss You My Buddy Smokey Robinson
Aug 20, 2025
-
Which Phrase Has The Most Positive Connotation
Aug 20, 2025
-
How Many Meters Are In 2 4 Km
Aug 20, 2025
-
How Much Do Contestants Make On Naked And Afraid
Aug 20, 2025
Related Post
Thank you for visiting our website which covers about Why Is Density A Derived Unit . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.