Atoms From Which Two Elements Would Form Ionic Bonds
Kalali
Apr 06, 2025 · 6 min read
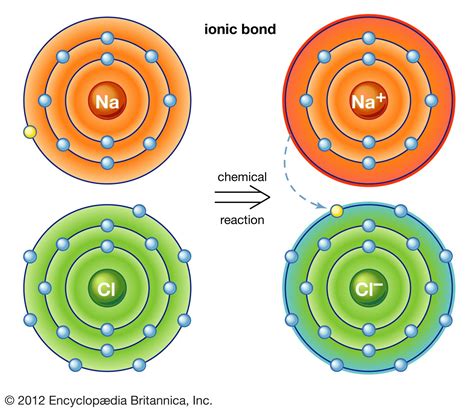
Table of Contents
Atoms From Which Two Elements Would Form Ionic Bonds? Understanding Ionic Bonding and Predicting Ionic Compounds
Ionic bonding, a fundamental concept in chemistry, describes the electrostatic attraction between oppositely charged ions. This powerful bond forms the foundation of countless compounds crucial to our daily lives, from the salt we use in cooking to the minerals that make up our bones. Understanding which elements readily form ionic bonds requires a grasp of atomic structure, electronegativity, and the principles governing electron transfer. This comprehensive guide delves into the intricacies of ionic bonding, providing a thorough exploration of the elements most likely to participate in this fascinating chemical interaction.
Understanding Atomic Structure and Electronegativity
Before diving into the specifics of ionic bonding, it's essential to review the basics of atomic structure and a crucial property called electronegativity. Atoms, the fundamental building blocks of matter, consist of a nucleus containing positively charged protons and neutral neutrons, surrounded by negatively charged electrons orbiting in shells or energy levels.
The number of protons defines an element's atomic number and dictates its chemical properties. Electrons, particularly those in the outermost shell (valence electrons), determine how an atom will interact with other atoms. Atoms strive for stability, often achieving it by acquiring a full outermost electron shell, mimicking the electron configuration of noble gases. This drive for stability is the driving force behind ionic bonding.
Electronegativity, a crucial concept in predicting bonding behavior, measures an atom's ability to attract electrons towards itself in a chemical bond. Elements with high electronegativity strongly attract electrons, while those with low electronegativity have a weaker pull. The difference in electronegativity between two atoms is a key predictor of the type of bond they will form – ionic, covalent, or metallic. A large difference in electronegativity leads to the transfer of electrons, resulting in ionic bonding.
The Formation of Ionic Bonds: A Detailed Look
Ionic bonds form when one atom readily loses electrons (becoming a positively charged cation) and another atom readily gains electrons (becoming a negatively charged anion). This electron transfer is driven by the significant electronegativity difference between the two atoms. The electrostatic attraction between the resulting oppositely charged ions holds the compound together.
Let's consider the classic example of sodium chloride (NaCl), or common table salt. Sodium (Na) has one valence electron, while chlorine (Cl) has seven. Sodium has a low electronegativity and readily loses its valence electron to achieve a stable octet (a full outer shell of eight electrons). Chlorine, with a high electronegativity, readily accepts this electron, completing its own octet.
This electron transfer results in a positively charged sodium ion (Na+) and a negatively charged chloride ion (Cl-). The strong electrostatic attraction between these oppositely charged ions forms the ionic bond in sodium chloride. The resulting crystal lattice structure of NaCl is a testament to the strength and orderliness of this bonding.
Key Characteristics of Ionic Compounds
- High melting and boiling points: The strong electrostatic forces between ions require significant energy to overcome, resulting in high melting and boiling points.
- Crystalline structure: Ionic compounds typically form a regular, repeating crystal lattice structure, reflecting the ordered arrangement of ions.
- Solubility in water: Many ionic compounds are soluble in water, as water molecules can effectively surround and separate the ions, reducing the electrostatic attraction between them.
- Conductivity in solution: When dissolved in water, ionic compounds conduct electricity because the free-moving ions can carry an electric current.
Predicting Ionic Bonds: Elements Involved
The likelihood of two elements forming an ionic bond is largely determined by their position on the periodic table. Generally, ionic bonds are most likely to form between:
-
Alkali metals (Group 1) and halogens (Group 17): Alkali metals have one valence electron and readily lose it to form a +1 cation. Halogens have seven valence electrons and readily gain one electron to form a -1 anion. The large electronegativity difference between these groups makes ionic bond formation highly favorable. Examples include NaCl, KCl, LiBr, and NaI.
-
Alkali earth metals (Group 2) and halogens (Group 17): Alkali earth metals have two valence electrons and form +2 cations. They can also form ionic bonds with halogens, resulting in compounds like MgCl2, CaF2, and BaBr2.
-
Alkali metals (Group 1) and oxygen (Group 16): Oxygen has six valence electrons and readily gains two electrons to form a -2 anion. Alkali metals can react with oxygen to form ionic oxides like Na2O, K2O, and Li2O.
-
Alkali earth metals (Group 2) and oxygen (Group 16): Alkali earth metals react with oxygen to form ionic oxides such as MgO, CaO, and BaO.
-
Transition metals and nonmetals: Transition metals, situated in the d-block of the periodic table, exhibit variable oxidation states (meaning they can form ions with different charges). They can form ionic compounds with a variety of nonmetals, often resulting in compounds with complex stoichiometries. Examples include FeCl3, CuO, and ZnS.
It's important to note that while these are common pairings, other combinations are possible. The key factor remains the significant difference in electronegativity between the two elements.
Factors Influencing Ionic Bond Strength
While electronegativity difference is the primary determinant, other factors influence the strength of an ionic bond:
-
Charge of the ions: Higher charges on the ions lead to stronger electrostatic attraction and a stronger ionic bond. For example, the bond in MgO (Mg2+ and O2-) is stronger than the bond in NaCl (Na+ and Cl-).
-
Size of the ions: Smaller ions result in a stronger ionic bond because the electrostatic forces are concentrated over a smaller distance.
-
Lattice energy: Lattice energy, the energy required to separate one mole of an ionic compound into its gaseous ions, is a direct measure of the ionic bond strength. Higher lattice energy indicates a stronger bond.
Examples of Ionic Compounds and Their Applications
Ionic compounds are ubiquitous in our daily lives. Here are some examples and their diverse applications:
-
Sodium chloride (NaCl): Table salt, essential for human health and widely used in food preservation and industrial processes.
-
Calcium carbonate (CaCO3): A major component of limestone, marble, and chalk; also used in antacids and construction materials.
-
Potassium chloride (KCl): Used as a fertilizer and in medical applications.
-
Magnesium oxide (MgO): Used as a refractory material (resistant to high temperatures), in insulation, and in medicine.
-
Iron(III) oxide (Fe2O3): The primary component of rust and used in pigments and magnetic materials.
Conclusion: The Importance of Understanding Ionic Bonding
Ionic bonding is a crucial concept in chemistry, governing the formation and properties of a vast array of compounds with diverse applications. Understanding the factors that influence ionic bond formation, particularly electronegativity and the positions of elements on the periodic table, allows us to predict which elements will readily form ionic bonds and the properties of the resulting compounds. This knowledge is essential for various fields, including materials science, medicine, agriculture, and environmental science. Further exploration of ionic bonding, including the study of crystal structures and lattice energy, provides deeper insights into the fascinating world of chemical bonding. Continued research in this area is vital for developing new materials and understanding complex chemical phenomena.
Latest Posts
Latest Posts
-
What Is 3 7 As A Decimal
Apr 08, 2025
-
How Many Parallel Sides Does A Rhombus Have
Apr 08, 2025
-
2 Out Of 6 Is What Percent
Apr 08, 2025
-
How Many Liters Are In 1000 Milliliters
Apr 08, 2025
-
How Many Grams Is 150 Mg
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about Atoms From Which Two Elements Would Form Ionic Bonds . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.