Ba Oh 2 Strong Or Weak
Kalali
Mar 13, 2025 · 5 min read
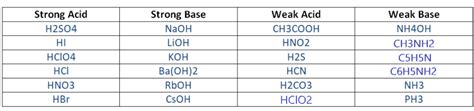
Table of Contents
Ba(OH)₂: Strong or Weak? Understanding its Properties and Applications
Barium hydroxide, Ba(OH)₂, is a strong base, a fact that significantly impacts its chemical behavior and applications. Understanding its strength and properties is crucial in various fields, from industrial processes to laboratory experiments. This comprehensive article delves into the details of Ba(OH)₂, exploring its strength as a base, its chemical properties, various applications, safety precautions, and common misconceptions.
Understanding the Strength of a Base
Before diving into the specifics of barium hydroxide, let's clarify what constitutes a strong base. A strong base is a base that completely dissociates into its ions (cations and anions) in an aqueous solution. This means that when dissolved in water, essentially all of the base molecules break apart into hydroxide ions (OH⁻) and the corresponding cation. This high concentration of hydroxide ions leads to a high pH, indicating a strongly alkaline solution. Conversely, a weak base only partially dissociates in water, resulting in a lower concentration of hydroxide ions and a less alkaline solution.
Ba(OH)₂: A Complete Dissociation
Barium hydroxide, when dissolved in water, undergoes complete dissociation, producing barium ions (Ba²⁺) and hydroxide ions (OH⁻):
Ba(OH)₂(aq) → Ba²⁺(aq) + 2OH⁻(aq)
This complete dissociation is the defining characteristic of a strong base. The equilibrium of this reaction strongly favors the products, meaning almost all the Ba(OH)₂ molecules break apart into ions. This high concentration of hydroxide ions is what makes Ba(OH)₂ a powerful base and a potent reactant in various chemical processes.
Chemical Properties of Barium Hydroxide
Beyond its strength as a base, Ba(OH)₂ exhibits several key chemical properties:
1. Reactivity with Acids:
As a strong base, Ba(OH)₂ readily reacts with acids in a neutralization reaction, forming water and a salt. For example, its reaction with hydrochloric acid (HCl) is:
Ba(OH)₂(aq) + 2HCl(aq) → BaCl₂(aq) + 2H₂O(l)
This reaction is highly exothermic, releasing a significant amount of heat.
2. Reactivity with Carbon Dioxide:
Barium hydroxide reacts with carbon dioxide (CO₂) in the air, forming barium carbonate (BaCO₃) and water:
Ba(OH)₂(aq) + CO₂(g) → BaCO₃(s) + H₂O(l)
This reaction is often used to detect the presence of CO₂. The formation of the white precipitate of barium carbonate is a clear indication of CO₂.
3. Thermal Decomposition:
Upon heating to high temperatures, barium hydroxide can decompose, losing water and forming barium oxide (BaO):
Ba(OH)₂(s) → BaO(s) + H₂O(g)
This decomposition highlights the hydroxide's instability at elevated temperatures.
4. Solubility:
While Ba(OH)₂ is considered a strong base due to its complete dissociation, its solubility in water is relatively low compared to other strong bases like sodium hydroxide (NaOH) or potassium hydroxide (KOH). Its solubility increases with temperature. This low solubility often limits its use in certain applications where high concentrations are required.
Applications of Barium Hydroxide
Despite its lower solubility compared to other strong bases, barium hydroxide finds applications in various fields:
1. Industrial Applications:
-
Sugar Refining: Ba(OH)₂ is used in the sugar refining industry to remove impurities from sugar beet juice. It forms insoluble compounds with impurities, allowing for their easy separation.
-
Water Treatment: In specific water treatment applications, barium hydroxide can be used to adjust the pH of water or to remove certain contaminants. However, due to the toxicity of barium compounds, its use is carefully controlled.
-
Chemical Synthesis: Ba(OH)₂ serves as a reactant in various chemical synthesis processes, often as a strong base catalyst or in neutralization reactions.
2. Laboratory Applications:
-
Titrations: In analytical chemistry, Ba(OH)₂ can be used as a titrant for acid-base titrations, especially for weak acids which require a strong base for accurate measurements. However, its low solubility often necessitates careful handling.
-
Preparation of other Barium Compounds: It serves as a starting material for the preparation of other barium compounds, taking advantage of its reactivity as a strong base.
-
Qualitative Analysis: Its reaction with CO₂ to produce a white precipitate of barium carbonate is used in qualitative analysis to detect the presence of CO₂.
Safety Precautions when Handling Barium Hydroxide
Barium hydroxide is a corrosive substance and is toxic if ingested or inhaled. Appropriate safety precautions must be taken when handling it:
-
Eye Protection: Always wear safety goggles or a face shield to protect your eyes from splashes.
-
Skin Protection: Wear gloves and appropriate clothing to prevent skin contact.
-
Respiratory Protection: If handling large quantities or in poorly ventilated areas, wear a respirator to prevent inhalation of dust or fumes.
-
Disposal: Dispose of barium hydroxide waste according to local regulations. Never dispose of it down the drain.
-
Emergency Response: In case of accidental ingestion, contact a poison control center or medical professional immediately. If it comes into contact with skin or eyes, flush the affected area with plenty of water for at least 15 minutes and seek medical attention.
Common Misconceptions about Ba(OH)₂
Several misconceptions surround barium hydroxide:
1. Confusing Strength and Solubility:
It is crucial to remember that the strength of a base refers to its complete dissociation, not its solubility. Ba(OH)₂ is a strong base because it completely dissociates in water, not because it's highly soluble.
2. Overestimating its Applicability:
While useful in specific applications, Ba(OH)₂'s low solubility limits its use in many areas where other strong bases are preferred due to their higher solubility and ease of handling.
3. Underestimating its Toxicity:
Barium compounds are toxic. Never underestimate the health risks associated with handling Ba(OH)₂. Always prioritize safety.
Conclusion
Barium hydroxide, Ba(OH)₂, is unequivocally a strong base due to its complete dissociation in water, resulting in a high concentration of hydroxide ions. Its properties and reactivity make it useful in various applications, from industrial processes to laboratory experiments. However, it's crucial to remember its low solubility and toxicity. Always handle it with appropriate safety precautions to avoid any health risks. A thorough understanding of its properties and limitations is essential for its safe and effective use. The key takeaway is that Ba(OH)₂'s strength as a base is a fundamental property, distinct from its solubility and must be considered alongside its toxicity for safe and effective usage.
Latest Posts
Latest Posts
-
How Many Inches Are In 27 Feet
Mar 13, 2025
-
What Is 20 25 As A Percent
Mar 13, 2025
-
Which Has More Protons Sulfur Or Iodine
Mar 13, 2025
-
How Many Centimeters Are In 69 Inches
Mar 13, 2025
-
How Many Cm In 23 Inches
Mar 13, 2025
Related Post
Thank you for visiting our website which covers about Ba Oh 2 Strong Or Weak . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
