Billiard Ball Model By John Dalton
Kalali
Mar 26, 2025 · 6 min read
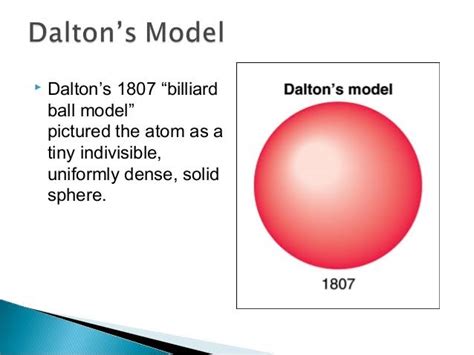
Table of Contents
The Billiard Ball Model: John Dalton's Revolutionary Contribution to Atomic Theory
John Dalton's billiard ball model, proposed in the early 1800s, marked a pivotal moment in the history of chemistry and our understanding of matter. Before Dalton, the concept of atoms was largely philosophical speculation. Dalton's model, while rudimentary by today's standards, provided the first concrete scientific framework for understanding the fundamental building blocks of matter, laying the foundation for modern atomic theory. This article delves deep into Dalton's billiard ball model, exploring its postulates, significance, limitations, and lasting impact on scientific thought.
The Dawn of Atomic Theory: Pre-Daltonian Ideas
Before Dalton's revolutionary ideas, the concept of atoms was primarily philosophical. Ancient Greek philosophers like Democritus and Leucippus proposed the existence of indivisible particles called "atomos," meaning "uncuttable." However, these ideas lacked experimental evidence and remained largely speculative. The alchemists of the Middle Ages, while pursuing the transmutation of elements, did not contribute significantly to a systematic understanding of atomic structure.
The groundwork for Dalton's model was partially laid by the work of scientists like Antoine Lavoisier and Joseph Proust. Lavoisier's meticulous experiments established the law of conservation of mass – matter cannot be created or destroyed, only transformed. Proust's law of definite proportions stated that a chemical compound always contains the same elements in the same proportions by mass. These laws provided crucial experimental support for the idea of discrete particles combining in fixed ratios to form compounds.
Dalton's Postulates: Defining the Billiard Ball Model
Dalton's atomic theory, presented in his seminal work A New System of Chemical Philosophy (1808), consisted of several key postulates that defined his famous "billiard ball" model:
1. All matter is made of atoms:
This seemingly simple statement was a radical departure from previous views. Dalton postulated that all matter, regardless of its form, was composed of indivisible and indestructible particles called atoms. This assertion provided a foundational principle for understanding the nature of substances.
2. All atoms of a given element are identical in mass and properties:
This postulate implied that atoms of a particular element were uniform and indistinguishable from one another. All atoms of oxygen, for example, were considered identical in mass and other characteristics. This uniformity, though later proven to be an oversimplification, was a critical component of the model.
3. Atoms of different elements have different masses and properties:
Dalton recognized that different elements were composed of different types of atoms, each possessing unique mass and properties. This distinction was crucial for understanding the diversity of substances found in nature.
4. Atoms combine in simple, whole-number ratios to form chemical compounds:
This postulate directly explained the law of definite proportions. Dalton suggested that atoms of different elements combine in specific whole-number ratios to create compounds. For instance, water (H₂O) always contains two hydrogen atoms for every one oxygen atom. This concept of combining ratios was a significant advance in understanding chemical reactions.
5. Atoms cannot be created or destroyed in chemical reactions:
This postulate reiterated Lavoisier's law of conservation of mass within the context of atomic theory. Dalton argued that atoms were neither created nor destroyed during chemical reactions; they merely rearranged themselves to form new substances.
The Billiard Ball Analogy: Simplicity and Limitations
The name "billiard ball model" aptly describes Dalton's vision of the atom. He visualized atoms as solid, indivisible spheres, similar to billiard balls, each possessing a unique mass and properties. The simplicity of this analogy allowed scientists to conceptualize atoms in a tangible way, facilitating the understanding of chemical reactions and the composition of substances.
However, the model's simplicity was also its major limitation. It did not account for the internal structure of the atom, the existence of subatomic particles like electrons, protons, and neutrons, or isotopes (atoms of the same element with different masses). These limitations would become apparent with further advancements in scientific understanding.
The Impact and Legacy of Dalton's Model
Despite its limitations, Dalton's billiard ball model had a profound impact on the development of chemistry and atomic theory. Its key contributions include:
- Providing a quantitative framework for chemistry: Dalton's model introduced the concept of atomic weight, allowing scientists to calculate relative atomic masses and formulate chemical formulas.
- Explaining fundamental chemical laws: The model successfully explained the laws of conservation of mass and definite proportions, providing a theoretical underpinning for these empirically observed laws.
- Stimulating further research: The model, though incomplete, laid the foundation for future atomic models, motivating scientists to probe deeper into the atom's structure and unravel its mysteries. It prompted scientists to develop experimental techniques to determine the relative atomic masses of elements.
The model facilitated the development of the periodic table, as scientists attempted to organize elements based on their relative atomic weights and chemical properties. Dmitri Mendeleev's periodic table, a monumental achievement in chemistry, owes a significant debt to Dalton's work.
Subsequent Developments and the Refinement of Atomic Theory
Dalton's model was gradually refined and superseded by more sophisticated models as new experimental techniques and discoveries emerged. J.J. Thomson's discovery of the electron in 1897 shattered the idea of the atom as an indivisible sphere. Thomson's "plum pudding" model depicted the atom as a positively charged sphere with negatively charged electrons embedded within it.
Ernest Rutherford's gold foil experiment in 1911 further revolutionized atomic theory. His experiment revealed the existence of a small, dense, positively charged nucleus at the center of the atom, surrounded by a cloud of orbiting electrons. This nuclear model significantly departed from Dalton's simple billiard ball model.
Niels Bohr's model, introduced in 1913, built upon Rutherford's work by incorporating principles of quantum mechanics. Bohr's model depicted electrons orbiting the nucleus in specific energy levels or shells. This model offered a more accurate explanation of atomic spectra and chemical bonding. Further developments led to the quantum mechanical model, which provides the most accurate and comprehensive description of the atom to date.
Dalton's Enduring Contribution
While superseded by more accurate models, Dalton's billiard ball model remains a landmark achievement in the history of science. Its simplicity and intuitive nature made it accessible to scientists and students alike, facilitating a deeper understanding of atomic theory. The model's emphasis on quantitative analysis and its successful explanation of fundamental chemical laws cemented its importance. Furthermore, the model highlights the iterative nature of scientific progress, with earlier models providing a foundation for more refined and accurate subsequent models. The legacy of Dalton's billiard ball model is a testament to the power of simple, yet groundbreaking, ideas in advancing scientific knowledge. The model's enduring relevance serves as a reminder that even seemingly simplistic models can profoundly impact scientific understanding, prompting further investigation and paving the way for more comprehensive theories. Understanding Dalton's work provides essential context for appreciating the complexity and sophistication of modern atomic theory.
Latest Posts
Latest Posts
-
What Percent Is 25 Of 50
Mar 29, 2025
-
How Many Pt In A Liter
Mar 29, 2025
-
Cuanto Es 46 Grados Fahrenheit En Centigrados
Mar 29, 2025
-
How Long Is 21 Cm In Inches
Mar 29, 2025
-
How To Recognize A Redox Reaction
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Billiard Ball Model By John Dalton . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
