How To Recognize A Redox Reaction
Kalali
Mar 29, 2025 · 6 min read
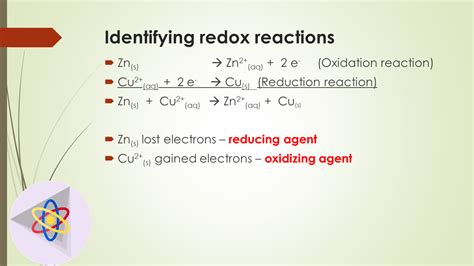
Table of Contents
How to Recognize a Redox Reaction: A Comprehensive Guide
Redox reactions, short for reduction-oxidation reactions, are fundamental chemical processes that underpin a vast array of natural phenomena and industrial applications. From rusting iron to photosynthesis, from battery operation to combustion, redox reactions are everywhere. Understanding how to identify them is crucial for anyone studying chemistry or related fields. This comprehensive guide will equip you with the knowledge and tools to confidently recognize redox reactions in various contexts.
Understanding the Fundamentals: Oxidation and Reduction
Before diving into recognition techniques, let's solidify our understanding of the core concepts: oxidation and reduction. These terms, often abbreviated as "redox," describe the transfer of electrons between chemical species.
Oxidation: This process involves the loss of electrons by an atom, molecule, or ion. When a substance is oxidized, its oxidation state (oxidation number) increases. Remember the mnemonic OIL RIG – Oxidation Is Loss, Reduction Is Gain of electrons.
Reduction: This process involves the gain of electrons by an atom, molecule, or ion. When a substance is reduced, its oxidation state decreases.
It's crucial to understand that oxidation and reduction always occur simultaneously. One substance cannot be oxidized without another being reduced, and vice-versa. This coupled nature is what defines a redox reaction.
Identifying Oxidation and Reduction: A Closer Look
Identifying oxidation and reduction often involves analyzing changes in oxidation states. Assigning oxidation states can be complex, but here are some key rules:
-
Free elements: The oxidation state of an atom in its elemental form is always 0. For example, the oxidation state of O<sub>2</sub> or Fe is 0.
-
Monatomic ions: The oxidation state of a monatomic ion is equal to its charge. For example, the oxidation state of Na<sup>+</sup> is +1, and Cl<sup>-</sup> is -1.
-
Oxygen: Oxygen usually has an oxidation state of -2, except in peroxides (like H<sub>2</sub>O<sub>2</sub>) where it's -1, and in compounds with fluorine (like OF<sub>2</sub>) where it's +2.
-
Hydrogen: Hydrogen usually has an oxidation state of +1, except in metal hydrides (like NaH) where it's -1.
-
Group 1 elements: Group 1 elements (alkali metals) always have an oxidation state of +1.
-
Group 2 elements: Group 2 elements (alkaline earth metals) always have an oxidation state of +2.
-
The sum of oxidation states: In a neutral molecule, the sum of the oxidation states of all atoms must be 0. In a polyatomic ion, the sum of oxidation states must equal the charge of the ion.
Recognizing Redox Reactions: Practical Techniques
Now that we've established the fundamentals, let's explore practical techniques for recognizing redox reactions:
1. Observing Changes in Oxidation States: The Most Reliable Method
This is the most direct and reliable method. By assigning oxidation states to all atoms in the reactants and products, you can definitively determine if a redox reaction has occurred. If any atom's oxidation state changes, a redox reaction is taking place. Let's illustrate this with an example:
Reaction: 2Fe + 3Cl<sub>2</sub> → 2FeCl<sub>3</sub>
Oxidation States:
- Reactants: Fe (0), Cl (0)
- Products: Fe (+3), Cl (-1)
Analysis: Iron's oxidation state increases from 0 to +3 (oxidation), while chlorine's oxidation state decreases from 0 to -1 (reduction). Since both oxidation and reduction occur, this is a redox reaction.
2. Identifying Electron Transfer: A Direct Approach
In some cases, the electron transfer is explicitly shown in the reaction equation. For instance:
Reaction: Zn + Cu<sup>2+</sup> → Zn<sup>2+</sup> + Cu
Here, zinc (Zn) loses two electrons to become Zn<sup>2+</sup> (oxidation), and copper(II) ion (Cu<sup>2+</sup>) gains two electrons to become Cu (reduction). The direct electron transfer clearly indicates a redox reaction.
3. Recognizing Common Redox Reactions: Patterns and Indicators
While changes in oxidation states are the ultimate proof, recognizing certain patterns can help you quickly identify many redox reactions:
-
Reactions with oxygen: Reactions where a substance reacts with oxygen, often leading to the formation of an oxide, are frequently redox reactions. For example, combustion reactions (burning) are classic redox reactions.
-
Reactions with halogens: Reactions involving halogens (F<sub>2</sub>, Cl<sub>2</sub>, Br<sub>2</sub>, I<sub>2</sub>) often involve redox processes. Halogens tend to gain electrons (reduction).
-
Reactions involving hydrogen: Some reactions involving hydrogen can be redox reactions. For example, the reaction between hydrogen and oxygen to form water is a redox reaction.
-
Single displacement reactions: These reactions, where one element replaces another in a compound, often involve redox processes. For example: Zn + 2HCl → ZnCl<sub>2</sub> + H<sub>2</sub>
-
Disproportionation reactions: In these reactions, the same element undergoes both oxidation and reduction. For example: 2Cu<sup>+</sup> → Cu<sup>2+</sup> + Cu
4. Using the Half-Reaction Method: A Powerful Tool for Complex Reactions
For more complex redox reactions, the half-reaction method is incredibly useful. This method involves separating the overall reaction into two half-reactions: one for oxidation and one for reduction. Balancing these half-reactions individually and then combining them provides a clear picture of the electron transfer.
Common Mistakes to Avoid
-
Misinterpreting oxidation states: Incorrectly assigning oxidation states can lead to misidentification. Pay close attention to the rules and practice assigning oxidation states regularly.
-
Ignoring spectator ions: Spectator ions (ions that do not participate in the reaction) can sometimes obscure the actual redox process. Focus on the species that undergo changes in oxidation states.
-
Confusing acid-base reactions with redox reactions: Acid-base reactions involve the transfer of protons (H<sup>+</sup>), not electrons. Don't mistake them for redox reactions.
-
Overlooking disproportionation: Remember that the same element can be both oxidized and reduced within a single reaction.
Applying your Knowledge: Real-World Examples
Let's solidify your understanding by examining some real-world examples:
-
Rusting of iron: Iron (Fe) reacts with oxygen (O<sub>2</sub>) and water (H<sub>2</sub>O) to form iron(III) oxide (Fe<sub>2</sub>O<sub>3</sub>), commonly known as rust. This is a classic example of a redox reaction, where iron is oxidized and oxygen is reduced.
-
Photosynthesis: Plants use sunlight to convert carbon dioxide (CO<sub>2</sub>) and water (H<sub>2</sub>O) into glucose (C<sub>6</sub>H<sub>12</sub>O<sub>6</sub>) and oxygen (O<sub>2</sub>). This process involves the reduction of carbon dioxide and the oxidation of water.
-
Combustion of fuels: Burning fuels like gasoline or natural gas involves the rapid oxidation of hydrocarbons in the presence of oxygen, releasing energy in the form of heat and light. This is a highly exothermic redox reaction.
-
Battery operation: Batteries operate based on redox reactions. Electrons flow from the anode (oxidation) to the cathode (reduction), generating an electric current.
Conclusion
Recognizing redox reactions is a crucial skill in chemistry. By understanding the fundamentals of oxidation and reduction, mastering techniques for identifying electron transfer and changes in oxidation states, and practicing with real-world examples, you'll gain the confidence to accurately identify and analyze these essential chemical processes. Remember to always focus on the changes in oxidation states—this is the definitive method for determining whether a given reaction is a redox reaction or not. Consistent practice will solidify your understanding and enable you to confidently tackle even complex redox reactions.
Latest Posts
Latest Posts
-
Convert 1 1 2 Ounces To Cups
Mar 31, 2025
-
Explain The Difference Between How Autotrophs And Heterotrophs Acquire Energy
Mar 31, 2025
-
How Do You Find The Volume Of A Solid Figure
Mar 31, 2025
-
What Is 20 Percent Of 6
Mar 31, 2025
-
3 Of 16 Is What Percent
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about How To Recognize A Redox Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
