Boiling Point Of Water In K
Kalali
Mar 30, 2025 · 5 min read
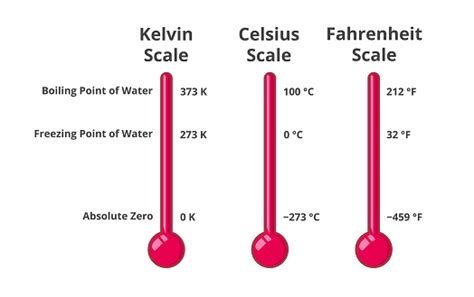
Table of Contents
- Boiling Point Of Water In K
- Table of Contents
- Boiling Point of Water in Kelvin: A Deep Dive into the Science and Applications
- Understanding the Kelvin Scale and Absolute Zero
- The Significance of Absolute Zero
- The Boiling Point of Water in Kelvin: A Precise Definition
- Factors Affecting the Boiling Point of Water
- 1. Atmospheric Pressure: A Dominant Influence
- 2. Impurities in Water: Subtle Effects
- 3. Isotopic Composition: A Minor Factor
- Applications of the Boiling Point of Water in Kelvin
- 1. Thermodynamics and Heat Transfer Calculations
- 2. Chemical Engineering and Process Control
- 3. Meteorology and Climate Science
- 4. Food Science and Culinary Arts
- 5. Material Science and Phase Transitions
- Advanced Concepts and Related Topics
- 1. Vapor Pressure and Equilibrium
- 2. Critical Point and Supercritical Fluids
- 3. Enthalpy of Vaporization
- Conclusion: The Fundamental Importance of 373.15 K
- Latest Posts
- Latest Posts
- Related Post
Boiling Point of Water in Kelvin: A Deep Dive into the Science and Applications
The boiling point of water, a seemingly simple concept, is fundamental to countless scientific principles and everyday applications. While we commonly express it in Celsius (°C) or Fahrenheit (°F), understanding it in Kelvin (K) provides a crucial perspective within the realm of thermodynamics and physics. This article delves deep into the boiling point of water in Kelvin, exploring its scientific basis, factors influencing it, and its significance across various fields.
Understanding the Kelvin Scale and Absolute Zero
Before delving into the boiling point of water specifically, it's essential to grasp the Kelvin scale itself. Unlike Celsius and Fahrenheit, which are relative scales based on the freezing and boiling points of water, the Kelvin scale is an absolute temperature scale. Its zero point, 0 K, represents absolute zero – the theoretical temperature at which all molecular motion ceases. This makes it a crucial scale in scientific calculations, especially those involving thermodynamics and gas laws.
The Significance of Absolute Zero
Absolute zero is a theoretical concept because it's practically impossible to reach. As temperatures approach absolute zero, substances exhibit unusual quantum mechanical effects. The Kelvin scale's foundation in absolute zero provides a consistent and unambiguous reference point for measuring temperature, avoiding the arbitrary zero points of relative scales.
The Boiling Point of Water in Kelvin: A Precise Definition
The boiling point of water at standard atmospheric pressure (1 atmosphere or 101.325 kPa) is 100°C. To convert this to Kelvin, we simply add 273.15 to the Celsius value:
100°C + 273.15 = 373.15 K
Therefore, the boiling point of water in Kelvin is 373.15 K. This precise value is crucial for accurate scientific calculations and experiments.
Factors Affecting the Boiling Point of Water
While 373.15 K represents the boiling point under standard conditions, several factors can influence this value:
1. Atmospheric Pressure: A Dominant Influence
Atmospheric pressure exerts a significant influence on the boiling point of water. At higher altitudes, where atmospheric pressure is lower, water boils at a lower temperature. Conversely, at higher pressures, such as in a pressure cooker, water boils at a higher temperature. This relationship is described by the Clausius-Clapeyron equation, a fundamental concept in thermodynamics.
2. Impurities in Water: Subtle Effects
The presence of dissolved impurities in water can subtly alter its boiling point. Generally, adding solutes like salts increases the boiling point, a phenomenon known as boiling point elevation. This is a colligative property, meaning it depends on the concentration of solute particles, not their identity.
3. Isotopic Composition: A Minor Factor
The isotopic composition of water molecules can also have a minuscule effect on the boiling point. Water molecules containing heavier isotopes of hydrogen (deuterium) or oxygen will have slightly higher boiling points than those containing lighter isotopes. This effect is relatively small but measurable with precise instrumentation.
Applications of the Boiling Point of Water in Kelvin
The boiling point of water in Kelvin plays a crucial role in numerous scientific and industrial applications:
1. Thermodynamics and Heat Transfer Calculations
In thermodynamics, the boiling point is a critical parameter in calculating heat transfer, enthalpy changes, and other thermodynamic properties. The Kelvin scale's absolute nature ensures consistency and accuracy in these calculations.
2. Chemical Engineering and Process Control
Chemical engineers utilize the boiling point of water in designing and controlling various processes, including distillation, evaporation, and sterilization. Understanding how pressure affects the boiling point is crucial for optimizing these processes.
3. Meteorology and Climate Science
The boiling point of water is essential in understanding atmospheric processes, including cloud formation, precipitation, and evaporation. Changes in atmospheric pressure and temperature directly influence the boiling point and consequently affect weather patterns.
4. Food Science and Culinary Arts
The boiling point of water is fundamental to cooking and food preservation. Understanding how pressure and altitude affect boiling point helps chefs adjust cooking times and techniques to achieve optimal results.
5. Material Science and Phase Transitions
The boiling point signifies a phase transition – from liquid to gas. Studying this transition provides insights into the properties of materials and their behavior under different conditions.
Advanced Concepts and Related Topics
Several advanced concepts are closely related to the boiling point of water in Kelvin:
1. Vapor Pressure and Equilibrium
The boiling point is the temperature at which the vapor pressure of water equals the surrounding atmospheric pressure. Understanding vapor pressure is essential in comprehending boiling and related phenomena.
2. Critical Point and Supercritical Fluids
At a specific temperature and pressure (the critical point), the distinction between liquid and gas phases disappears. Understanding supercritical fluids, which exist beyond the critical point, is important in various industrial processes.
3. Enthalpy of Vaporization
The enthalpy of vaporization is the energy required to change one mole of liquid water to water vapor at its boiling point. This value is crucial in thermodynamic calculations and process design.
Conclusion: The Fundamental Importance of 373.15 K
The boiling point of water at 373.15 K serves as a fundamental constant in numerous scientific disciplines and practical applications. Understanding this value, the factors influencing it, and its implications is essential for anyone working in fields involving thermodynamics, chemistry, engineering, or meteorology. The Kelvin scale's absolute nature provides a precise and consistent framework for studying this critical physical phenomenon and its wide-ranging consequences. From culinary arts to advanced chemical engineering, the seemingly simple act of water boiling holds profound scientific significance, underpinned by the precise temperature of 373.15 K. Further research into the nuanced effects of pressure, impurities, and isotopic composition continues to refine our understanding of this crucial physical constant, advancing our knowledge across diverse scientific fields. The ongoing exploration of the boiling point of water, expressed elegantly in Kelvin, highlights its enduring relevance in our understanding of the natural world and its applications in human endeavors.
Latest Posts
Latest Posts
-
What Role Do Producers Play In The Carbon Cycle
Apr 02, 2025
-
How Does A Simple Machine Make Work Easier
Apr 02, 2025
-
How Many Centimeters Are In 42 Inches
Apr 02, 2025
-
Adding Integers With The Same Sign
Apr 02, 2025
-
5 To The Power Of 1
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Boiling Point Of Water In K . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
