Ch3 Ch2 Ch2 Ch2 Ch2 Ch2 Ch3
Kalali
Jun 16, 2025 · 2 min read
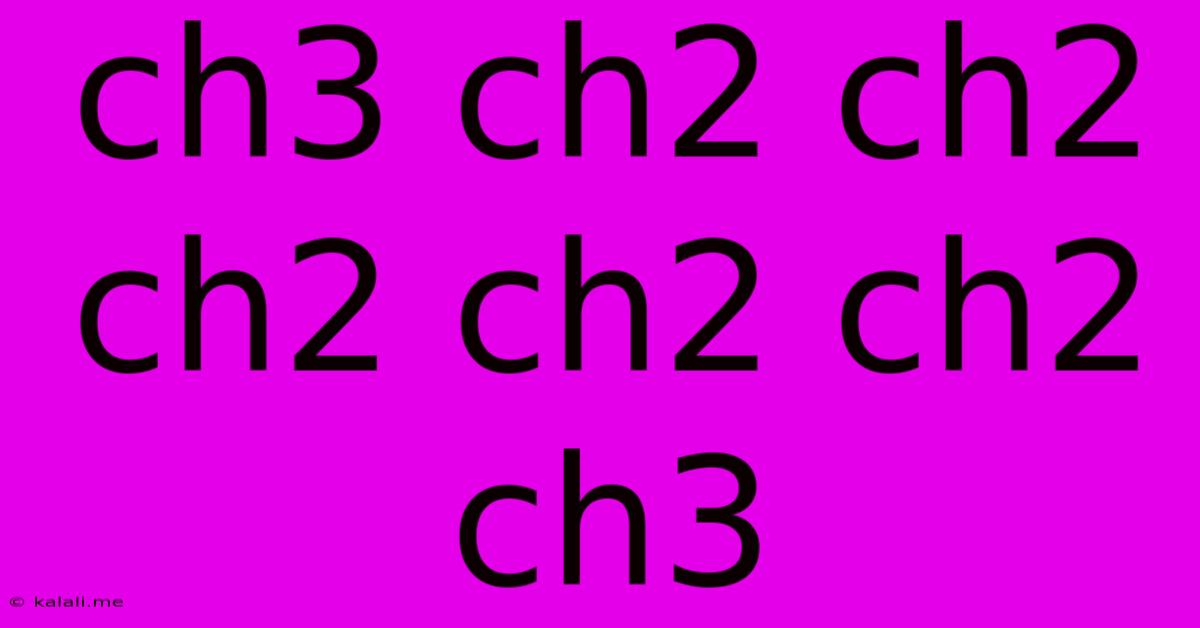
Table of Contents
Deciphering the Carbon Chain: CH3CH2CH2CH2CH2CH2CH3
This article delves into the organic compound represented by the chemical formula CH3CH2CH2CH2CH2CH2CH3, exploring its structure, properties, and applications. Understanding this seemingly simple molecule provides a foundational understanding of alkane chemistry and its relevance in various fields. We'll cover its IUPAC nomenclature, isomerism, physical properties, and potential uses.
What is CH3CH2CH2CH2CH2CH2CH3?
CH3CH2CH2CH2CH2CH2CH3 is the condensed structural formula for heptane, a saturated hydrocarbon belonging to the alkane family. Alkanes are characterized by their single carbon-carbon bonds, making them relatively unreactive compared to other hydrocarbon classes. The formula shows a linear chain of seven carbon atoms, each bonded to the maximum number of hydrogen atoms.
IUPAC Nomenclature and Isomerism
The systematic name, heptane, is derived from the IUPAC (International Union of Pure and Applied Chemistry) nomenclature system. The prefix "hept-" indicates seven carbon atoms, and the suffix "-ane" denotes its status as an alkane.
Importantly, heptane, with its straight chain structure, is only one possible isomer. Isomers are molecules with the same molecular formula but different structural arrangements. For example, several branched-chain isomers exist for heptane, each with unique physical and chemical properties. Understanding isomerism is crucial in organic chemistry, as it directly impacts the molecule's behaviour.
Physical Properties of Heptane
Heptane, like other alkanes, exhibits several key physical properties:
- State: At room temperature and pressure, heptane is a colorless liquid.
- Solubility: It's non-polar and therefore insoluble in water but readily dissolves in non-polar solvents like other hydrocarbons.
- Boiling Point: Its boiling point is relatively high compared to smaller alkanes, reflecting the stronger intermolecular forces (London dispersion forces) between its longer carbon chains.
- Density: Heptane is less dense than water, meaning it will float on water.
- Flammability: It's highly flammable, a characteristic shared by most alkanes.
Applications of Heptane
Heptane finds applications in several industries:
- Solvent: Due to its non-polar nature and ability to dissolve various non-polar substances, heptane is used as a solvent in various industrial processes, including cleaning and extraction.
- Component in Fuels: Heptane is a component of gasoline and other fuels, contributing to their combustion properties. Its octane rating is relevant in determining the fuel's performance.
- Laboratory Reagent: It serves as a valuable reagent in various laboratory settings for organic synthesis and analysis.
- Calibration Standard: In analytical chemistry, heptane is used as a calibration standard for gas chromatography and other analytical techniques.
Conclusion:
CH3CH2CH2CH2CH2CH2CH3, or heptane, is a fundamental example of an alkane. Understanding its structure, isomerism, and properties is essential for comprehending the broader field of organic chemistry. Its various applications highlight the importance of alkanes in different sectors, from fuels and solvents to laboratory research. Further exploration of its branched isomers and reactivity opens up even richer insights into the fascinating world of organic molecules.
Latest Posts
Latest Posts
-
How To Create Clickable Image In Html
Jun 16, 2025
-
What Are The Factors Of 121
Jun 16, 2025
-
What Is A Theme Of The Passage
Jun 16, 2025
-
A Company That Provides Access To The Internet
Jun 16, 2025
-
Which Word Is Closest In Meaning To The Underlined Word
Jun 16, 2025
Related Post
Thank you for visiting our website which covers about Ch3 Ch2 Ch2 Ch2 Ch2 Ch2 Ch3 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.