Do Electrolytic Cells Have Salt Bridges
Kalali
Apr 07, 2025 · 5 min read
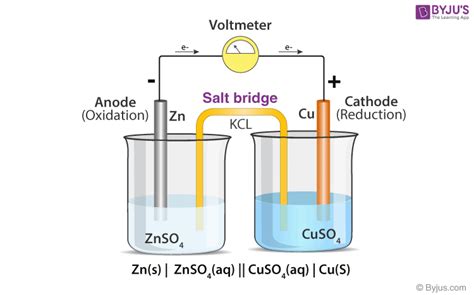
Table of Contents
Do Electrolytic Cells Have Salt Bridges? A Comprehensive Exploration
Electrolytic cells and galvanic (voltaic) cells are both electrochemical cells, but they operate under fundamentally different principles. A key difference lies in their need for a salt bridge. While galvanic cells typically require a salt bridge, the question of whether electrolytic cells need one is more nuanced and deserves a thorough exploration. This article will delve into the intricacies of electrolytic cells, examining the role of salt bridges and exploring alternative mechanisms for maintaining electrical neutrality.
Understanding Electrolytic Cells
Electrolytic cells are electrochemical cells that drive non-spontaneous redox reactions using an external electrical source. Unlike galvanic cells, which generate electricity from spontaneous reactions, electrolytic cells consume electrical energy to force a chemical change. This process is known as electrolysis.
Key components of an electrolytic cell include:
- Electrodes: Two conductive electrodes, an anode (positive electrode) and a cathode (negative electrode), are immersed in an electrolyte solution.
- Electrolyte: An electrolyte is a substance containing freely mobile ions, usually an ionic solution or molten salt, that allows the flow of electric current.
- External Power Source: A battery or other power source provides the electrical energy needed to drive the non-spontaneous redox reaction.
The process involves oxidation at the anode (electrons are lost) and reduction at the cathode (electrons are gained). The external power source forces electrons to flow from the anode to the cathode, opposite to the direction of spontaneous electron flow in a galvanic cell.
The Role of Ion Movement
The movement of ions within the electrolyte is crucial for maintaining electrical neutrality and allowing the redox reactions to proceed. As electrons flow through the external circuit, ions migrate within the electrolyte to balance the charges building up at the electrodes. At the anode, oxidation produces positive ions (cations), which migrate towards the cathode. Conversely, reduction at the cathode consumes electrons, creating negative ions (anions) that migrate towards the anode.
The Salt Bridge Debate: Do Electrolytic Cells Need Them?
The primary function of a salt bridge in a galvanic cell is to maintain electrical neutrality by allowing the flow of ions between the half-cells. This prevents the build-up of charge that would halt the cell's operation. However, the situation is different in electrolytic cells.
The short answer is: Not necessarily. While a salt bridge can be used in an electrolytic cell, it's not always essential. The crucial factor is the ability to maintain electrical neutrality within the electrolyte.
Why a salt bridge is often unnecessary:
- Single Compartment Cells: Many electrolytic cells are designed as single-compartment cells. The electrolyte solution surrounds both electrodes, allowing for free ion movement between the anode and cathode regions. This direct ion migration effectively fulfills the role of a salt bridge, preventing charge buildup. Examples include the electrolysis of water and the electrolysis of molten salts.
- Sufficient Ion Mobility: If the electrolyte has a high concentration of ions with good mobility, the inherent ion movement within the electrolyte is sufficient to balance the charges generated at the electrodes. A salt bridge adds unnecessary complexity.
- Electrolyte Composition: The nature of the electrolyte itself plays a significant role. Some electrolytes readily facilitate ion migration without needing external assistance from a salt bridge.
When a salt bridge might be beneficial:
- Preventing Mixing of Reactants: In some electrolytic setups, it may be necessary to prevent the direct mixing of reactants generated at the anode and cathode. A salt bridge, separating the two electrode compartments, can serve this purpose, preventing unwanted side reactions.
- Specific Electrolyte Requirements: Certain electrolytes might have limitations in their ion mobility, potentially requiring a salt bridge to facilitate faster charge neutralization.
- Improved Efficiency: In some cases, the use of a salt bridge could enhance the efficiency of the electrolytic process by optimizing ion transport and minimizing unwanted reactions.
Alternatives to Salt Bridges in Electrolytic Cells
Instead of a salt bridge, other methods ensure charge neutrality in electrolytic cells:
- Porous Membrane or Diaphragm: A porous membrane or diaphragm can separate the anode and cathode compartments, allowing ion transport while preventing direct mixing of reactants. This approach combines the benefits of compartmentalization with efficient ion transfer.
- Ion-Exchange Membrane: These specialized membranes selectively allow the passage of specific ions, further controlling the chemical environment at each electrode and optimizing the electrolytic process.
- Direct Ion Migration: As previously mentioned, if the electrolyte has sufficient ionic conductivity and the cell design allows for free ion movement, direct ion migration is the primary mechanism for maintaining electrical neutrality, eliminating the need for any additional components.
Practical Examples Illustrating the Absence of Salt Bridges
Let's consider some real-world examples where electrolytic cells successfully operate without a salt bridge:
- Electrolysis of Water: In the electrolysis of water, a single electrolyte solution (often diluted sulfuric acid or sodium hydroxide) surrounds both electrodes. Ions from the electrolyte readily migrate to balance the charges generated during oxidation and reduction, making a salt bridge unnecessary.
- Electrolysis of Molten Salts: The electrolysis of molten salts, like sodium chloride, involves no solvent. The molten salt itself serves as the electrolyte, providing ample ions for charge balancing. Again, a salt bridge is redundant.
- Electroplating: Many electroplating processes use a single electrolyte bath surrounding both electrodes, relying on the electrolyte's conductivity and ion mobility to maintain charge balance without a salt bridge.
Conclusion: Context Matters
The need for a salt bridge in an electrolytic cell is not absolute. Unlike galvanic cells, where salt bridges are generally essential, electrolytic cells often operate effectively without them, particularly in single-compartment setups with highly conductive electrolytes. The choice of whether or not to include a salt bridge depends on factors like the specific electrolyte used, the desired cell design, and the need to prevent unwanted mixing of reactants. The key principle is ensuring efficient charge neutralization and enabling the smooth progress of the electrolytic process. Understanding these factors allows for the optimal design and operation of electrolytic cells for various applications. Therefore, while a salt bridge can sometimes be beneficial, it is certainly not a universal requirement for all electrolytic cells. The most important consideration remains maintaining electrical neutrality, and many methods exist to achieve this without a salt bridge.
Latest Posts
Latest Posts
-
Cuanto Es 8 Onzas En Ml
Apr 09, 2025
-
What Is 75 Percent Of 12
Apr 09, 2025
-
What Is The Reciprocal Of 5 6
Apr 09, 2025
-
13 Out Of 40 As A Percentage
Apr 09, 2025
-
How Many Hours Is 96 Minutes
Apr 09, 2025
Related Post
Thank you for visiting our website which covers about Do Electrolytic Cells Have Salt Bridges . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.