Double Displacement Reaction Examples In Real Life
Kalali
Mar 28, 2025 · 5 min read
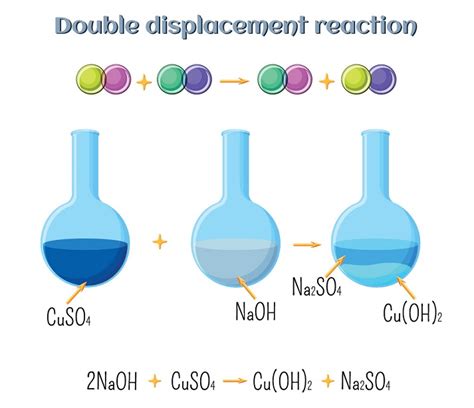
Table of Contents
Double Displacement Reactions: Everyday Encounters with Chemistry
Double displacement reactions, also known as double replacement reactions or metathesis reactions, are a fundamental type of chemical reaction where two compounds exchange ions or molecules to form two new compounds. This seemingly simple process underlies a surprising number of everyday occurrences, from the production of essential chemicals to everyday household tasks. This article delves into the fascinating world of double displacement reactions, providing numerous real-life examples and explaining the underlying chemistry.
Understanding the Mechanism of Double Displacement Reactions
Before exploring the applications, let's briefly review the mechanism. A general representation of a double displacement reaction is:
AB + CD → AD + CB
Where A and C are cations (positively charged ions) and B and D are anions (negatively charged ions). The reaction proceeds because the newly formed compounds (AD and CB) are more stable or less soluble than the reactants (AB and CD). This stability often stems from the formation of a precipitate (an insoluble solid), a gas, or water.
Key Drivers of Double Displacement Reactions
Several factors influence whether a double displacement reaction will occur:
- Solubility: The solubility of the products determines if a precipitate will form. If one or both products are insoluble in the solvent (usually water), they will precipitate out of the solution, driving the reaction forward. Solubility rules are crucial in predicting the outcome of these reactions.
- Formation of a Gas: The production of a gas, like carbon dioxide or hydrogen sulfide, is another driving force. The escape of the gas from the solution shifts the equilibrium to favor product formation.
- Formation of Water: The formation of water, a very stable molecule, is a powerful driving force. Neutralization reactions between acids and bases are classic examples of this.
Real-Life Examples of Double Displacement Reactions
Now, let's explore a wide range of real-life examples, categorized for clarity.
1. Everyday Household Applications:
-
Baking Soda and Vinegar: The classic volcano science experiment is a prime example. Baking soda (sodium bicarbonate, NaHCO₃) reacts with vinegar (acetic acid, CH₃COOH) in a double displacement reaction:
NaHCO₃ + CH₃COOH → CH₃COONa + H₂O + CO₂
The products are sodium acetate, water, and carbon dioxide gas (the bubbles in the volcano). This reaction demonstrates the formation of a gas and water as driving forces.
-
Antacids: Many antacids utilize double displacement reactions to neutralize stomach acid (hydrochloric acid, HCl). For example, magnesium hydroxide (Mg(OH)₂) in milk of magnesia reacts with HCl:
Mg(OH)₂ + 2HCl → MgCl₂ + 2H₂O
This produces magnesium chloride and water, relieving heartburn by neutralizing the acid. Here, the formation of water is the key driving force.
-
Soap Making (Saponification): The process of soap making involves a double displacement reaction between a fat or oil (a triglyceride) and a strong base like sodium hydroxide (NaOH). This reaction breaks down the fat into glycerol and soap (fatty acid salts).
2. Industrial Applications:
-
Production of Salts: Many industrial processes rely on double displacement reactions to produce various salts. For example, the production of silver chloride (AgCl), used in photography, involves reacting silver nitrate (AgNO₃) with sodium chloride (NaCl):
AgNO₃ + NaCl → AgCl + NaNO₃
The insoluble silver chloride precipitates out of the solution.
-
Water Softening: Water softening often involves a double displacement reaction using ion exchange resins. These resins exchange hard water ions (like calcium and magnesium) for softer sodium ions, improving water quality.
-
Mineral Processing: Double displacement reactions play a significant role in various mineral processing techniques, such as the precipitation of valuable metals from solutions using specific reagents.
3. Environmental Processes:
-
Acid Rain and Limestone: Acid rain, containing sulfuric acid (H₂SO₄), reacts with limestone (calcium carbonate, CaCO₃) in a double displacement reaction:
CaCO₃ + H₂SO₄ → CaSO₄ + H₂O + CO₂
This reaction leads to the deterioration of limestone structures and contributes to environmental damage. The formation of water and carbon dioxide drives this reaction.
-
Ocean Acidification: The absorption of carbon dioxide from the atmosphere by the ocean leads to the formation of carbonic acid (H₂CO₃), which reacts with carbonate ions (CO₃²⁻) in seawater. This reaction reduces the availability of carbonate ions for marine organisms like corals to build their shells and skeletons.
4. Biological Processes:
-
Blood Buffering System: The blood buffering system utilizes a series of double displacement reactions involving carbonic acid (H₂CO₃) and bicarbonate ions (HCO₃⁻) to maintain a stable pH level.
-
Enzyme-Substrate Interactions: While not strictly double displacement, some enzyme-catalyzed reactions involve the exchange of groups between molecules, bearing resemblance to the double displacement mechanism.
Predicting the Outcome of Double Displacement Reactions: Solubility Rules
Accurately predicting whether a double displacement reaction will occur requires an understanding of solubility rules. These rules outline the solubility of common ionic compounds in water. If a product is insoluble (precipitates), the reaction is favored. Here are some key solubility rules:
-
Generally Soluble: Most nitrates (NO₃⁻), acetates (CH₃COO⁻), and alkali metal salts (Li⁺, Na⁺, K⁺, Rb⁺, Cs⁺) are soluble.
-
Generally Insoluble: Most carbonates (CO₃²⁻), phosphates (PO₄³⁻), sulfides (S²⁻), and hydroxides (OH⁻) are insoluble (except for those of alkali metals and ammonium).
-
Exceptions: There are exceptions to these general rules. For instance, silver acetate is less soluble than most acetates.
Beyond the Basics: Limitations and Considerations
While the AB + CD → AD + CB representation simplifies the reaction, several factors can influence the outcome in real-world scenarios:
-
Complex Ion Formation: The formation of complex ions can alter the solubility of products and affect the overall reaction.
-
Reaction Kinetics: Some double displacement reactions are slow, while others are fast. Reaction kinetics depend on factors like temperature and concentration.
-
Equilibrium: Many double displacement reactions are reversible. The equilibrium position determines the extent of the reaction.
Conclusion: The Ubiquity of Double Displacement Reactions
Double displacement reactions are far more prevalent in our everyday lives than many realize. From the simple act of taking an antacid to the large-scale industrial processes, these reactions play a crucial role in shaping our world. Understanding the underlying principles, including solubility rules and driving forces, allows us to appreciate the intricate chemistry that governs countless everyday phenomena. Furthermore, this knowledge can inform the development of new technologies and processes across various industries. The study of double displacement reactions provides a fascinating window into the dynamic world of chemical transformations that constantly surround us.
Latest Posts
Latest Posts
-
How Many Inches Is 30 Ft
Mar 31, 2025
-
What Is The Boiling Point Of Saltwater
Mar 31, 2025
-
What Are The 2 Parts Of A Solution
Mar 31, 2025
-
What Are The Chemical Equations Of Photosynthesis And Cellular Respiration
Mar 31, 2025
-
What Is 57 Celsius In Fahrenheit
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Double Displacement Reaction Examples In Real Life . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
