What Is The Boiling Point Of Saltwater
Kalali
Mar 31, 2025 · 6 min read
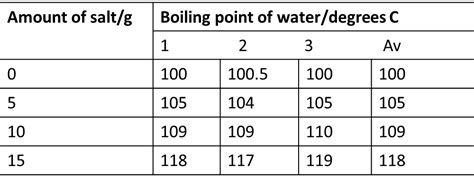
Table of Contents
What is the Boiling Point of Saltwater? A Deep Dive into Salinity and Temperature
The boiling point of water, a seemingly simple concept, becomes significantly more nuanced when considering the presence of dissolved salts. Understanding the boiling point of saltwater is crucial in various fields, from culinary arts and marine biology to industrial processes and even geology. This comprehensive guide delves into the science behind saltwater's elevated boiling point, exploring the factors influencing it and its practical implications.
Understanding the Basics: Pure Water vs. Saltwater
The boiling point of pure water at standard atmospheric pressure (1 atmosphere or 101.325 kPa) is precisely 100 degrees Celsius (212 degrees Fahrenheit). This temperature represents the point at which the vapor pressure of the liquid water equals the atmospheric pressure, allowing water molecules to transition to the gaseous phase (steam).
However, introducing salt (sodium chloride, NaCl) to water alters this fundamental property. Saltwater, a solution of water and dissolved salts, boasts a higher boiling point than pure water. This phenomenon is directly linked to colligative properties, properties that depend on the concentration of solute particles in a solution, rather than the identity of the solute itself.
Colligative Properties: The Key to Elevated Boiling Point
Several colligative properties influence the boiling point of a solution. The most significant in the case of saltwater is boiling point elevation. This property states that adding a non-volatile solute (like salt) to a solvent (like water) increases the boiling point of the solution.
The underlying mechanism is straightforward. Salt dissociates into ions (Na+ and Cl-) in water. These ions disrupt the water molecules' interaction, hindering their ability to escape into the gaseous phase. To achieve boiling, the solution must reach a higher temperature where the vapor pressure of the water molecules overcomes the increased intermolecular forces and atmospheric pressure.
Factors Affecting the Boiling Point of Saltwater
While the principle is relatively simple, several factors influence the precise boiling point of saltwater:
1. Salinity: The Concentration of Salt
The most significant factor determining the boiling point of saltwater is its salinity, representing the total amount of dissolved salts in the water. Higher salinity translates to a higher boiling point. This relationship isn't perfectly linear, but generally, the increase in boiling point is proportional to the concentration of dissolved salts. A highly saline solution like seawater will boil at a noticeably higher temperature than a solution with only a small amount of dissolved salt.
2. Type of Salt: Different Salts, Different Effects
While sodium chloride is the most common salt in seawater, other salts contribute to overall salinity. Different salts dissociate into varying numbers of ions. For example, magnesium chloride (MgCl2) dissociates into three ions (one Mg2+ and two Cl-), while sodium chloride (NaCl) only dissociates into two (one Na+ and one Cl-). The number of ions affects the magnitude of boiling point elevation. A solution containing MgCl2 at the same concentration as NaCl will exhibit a greater boiling point elevation.
3. Pressure: Atmospheric Influence
Atmospheric pressure exerts a significant impact on the boiling point of any liquid, including saltwater. At higher altitudes, where atmospheric pressure is lower, the boiling point of saltwater (and pure water) decreases. Conversely, at higher pressures (like in a pressure cooker), the boiling point increases. This effect is independent of salinity but interacts with it. The combined effect of high salinity and high pressure leads to a very significant increase in boiling point.
4. Temperature: A Dynamic Relationship
While temperature is the outcome of the boiling process, it also indirectly influences the boiling point. As the temperature of the saltwater increases, its density changes slightly. This change in density can subtly influence the vapor pressure and therefore the boiling point. However, the impact is typically minor compared to the effects of salinity and pressure.
Calculating the Boiling Point of Saltwater: Approximations and Limitations
Precisely calculating the boiling point of saltwater requires sophisticated thermodynamic models that account for the complex interactions between water molecules and ions. However, approximate calculations can be made using simplified equations based on the concept of boiling point elevation.
One commonly used approximation involves the Duhring's rule, which relates the boiling point of a solution to the boiling point of the pure solvent. However, Duhring's rule is an empirical relationship and its accuracy depends heavily on the specific solute and the range of concentrations considered.
Other approximations utilize the molality of the solution (moles of solute per kilogram of solvent) and a constant known as the ebullioscopic constant (Kb) for water. The equation is:
ΔTb = Kb * m * i
where:
- ΔTb is the boiling point elevation
- Kb is the ebullioscopic constant for water (approximately 0.512 °C/m)
- m is the molality of the solution
- i is the van't Hoff factor (the number of ions produced per formula unit of solute)
This equation provides a reasonable estimation, particularly for solutions with relatively low concentrations of salt. However, it becomes less accurate at higher concentrations due to deviations from ideal behavior.
Practical Implications of Saltwater's Boiling Point
The higher boiling point of saltwater has several significant practical applications:
-
Cooking: Cooking pasta or other foods in saltwater requires a slightly higher temperature compared to using freshwater. This difference is often negligible for casual cooking but becomes relevant in industrial food processing where precise temperature control is crucial.
-
Marine Biology: Understanding the boiling point of seawater is essential in marine research and aquaculture. Processes involving heating seawater, such as desalination or sterilization, need to account for the elevated boiling point.
-
Industrial Processes: Industries utilizing saltwater in their processes, like desalination plants or power generation using seawater cooling systems, require precise knowledge of the boiling point to optimize their operations and prevent equipment damage.
-
Geology: The boiling point of saltwater plays a role in geological processes such as geothermal energy generation and the formation of certain mineral deposits. Understanding how salinity affects boiling point helps researchers model these complex systems.
Conclusion: A Deeper Understanding of a Fundamental Property
The boiling point of saltwater is more than just a simple scientific curiosity. It’s a fundamental property with far-reaching implications across diverse fields. While calculating the precise boiling point can be complex, understanding the basic principles of colligative properties and the factors influencing boiling point elevation is crucial for anyone working with saltwater solutions. This understanding is key to optimizing processes, ensuring safety, and furthering research in areas ranging from culinary arts to complex geological systems. Further research into the nuances of salt-water interactions is essential for precise predictions and more accurate modeling of various systems involving saltwater. The information presented in this article serves as a foundation for continued exploration of this fascinating and impactful scientific topic.
Latest Posts
Latest Posts
-
How Many Feet Is 87 In
Apr 01, 2025
-
Number Of Energy Levels In Oxygen
Apr 01, 2025
-
What Is The Wavelength Of A 2 99 Hz Wave
Apr 01, 2025
-
36 Is 30 Percent Of What Number
Apr 01, 2025
-
How Many Hours Is 800 Minutes
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Is The Boiling Point Of Saltwater . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
