Electronegativity Increases As The Size Of The Atom Increases
Kalali
Apr 06, 2025 · 5 min read
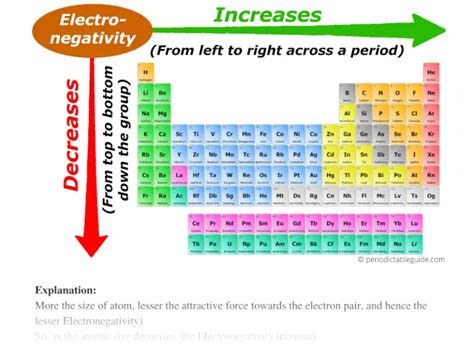
Table of Contents
Electronegativity: A Deeper Dive into Atomic Size and Electron Attraction
The statement "electronegativity increases as the size of the atom increases" is incorrect. In fact, the opposite is true: electronegativity generally decreases as the atomic size increases. This fundamental concept in chemistry governs the behavior of atoms in molecules and is crucial for understanding bonding, reactivity, and the properties of compounds. This article will delve into the intricacies of electronegativity, exploring its relationship with atomic size, periodic trends, and the implications for chemical behavior.
Understanding Electronegativity
Electronegativity quantifies an atom's ability to attract shared electrons in a chemical bond. A higher electronegativity value indicates a stronger pull on electrons. This attraction arises from the interplay of several factors, primarily the effective nuclear charge and the atomic radius.
Effective Nuclear Charge: The effective nuclear charge represents the net positive charge experienced by the valence electrons. It's the difference between the number of protons in the nucleus (which attracts electrons) and the number of inner electrons (which shield the valence electrons from the full positive charge of the nucleus). A higher effective nuclear charge leads to a stronger attraction for electrons, hence higher electronegativity.
Atomic Radius: The atomic radius refers to the distance from the nucleus to the outermost electrons. A larger atomic radius implies that the valence electrons are farther from the nucleus, experiencing a weaker pull. Therefore, a larger atomic radius correlates with lower electronegativity.
The Inverse Relationship Between Electronegativity and Atomic Size
The relationship between electronegativity and atomic size is inversely proportional. As the atomic size increases (moving down a group in the periodic table), the valence electrons are further from the nucleus, resulting in a weaker attractive force. Consequently, electronegativity decreases.
Consider the alkali metals (Group 1). Lithium (Li) has a smaller atomic radius and higher electronegativity than Cesium (Cs). This is because the valence electron in Li is closer to the nucleus, experiencing a stronger effective nuclear charge compared to the valence electron in Cs, which is shielded by more inner electron shells.
Similarly, moving across a period (from left to right), the atomic radius generally decreases while the effective nuclear charge increases. This simultaneous trend results in an increase in electronegativity. Fluorine (F), the most electronegative element, has a smaller atomic radius and a higher effective nuclear charge than Lithium.
Periodic Trends in Electronegativity
Electronegativity exhibits clear periodic trends that reflect the interplay of atomic size and effective nuclear charge:
- Across a Period (Left to Right): Electronegativity increases. This is because the number of protons increases, leading to a higher effective nuclear charge, while the atomic radius decreases.
- Down a Group (Top to Bottom): Electronegativity decreases. This is because the atomic radius increases, placing the valence electrons further from the nucleus, weakening the attractive force.
These trends are readily apparent when examining the electronegativity values of elements on the periodic table. The most electronegative elements are found in the upper right corner (excluding noble gases), while the least electronegative elements reside in the lower left corner.
Factors Affecting Electronegativity Beyond Atomic Size
While atomic size is a primary determinant of electronegativity, other factors also contribute:
-
Shielding Effect: Inner electrons shield the valence electrons from the full nuclear charge. Greater shielding reduces the effective nuclear charge and thus, electronegativity. This effect is prominent as you move down a group, where the number of inner electron shells increases.
-
Nuclear Charge: The number of protons in the nucleus directly impacts the attractive force on electrons. A higher nuclear charge leads to a greater pull and higher electronegativity.
-
Electron Configuration: The specific arrangement of electrons influences the shielding effect and the overall attraction between the nucleus and valence electrons. For instance, elements with filled or half-filled subshells (e.g., noble gases and some transition metals) exhibit slightly different electronegativity trends due to increased stability.
Applications and Implications of Electronegativity
Electronegativity is not merely an abstract concept; it has profound implications in various areas of chemistry:
-
Predicting Bond Polarity: The difference in electronegativity between two bonded atoms determines the polarity of the bond. A large electronegativity difference results in a polar covalent bond (e.g., H-Cl), where electrons are unequally shared. A small difference leads to a less polar or nonpolar covalent bond (e.g., C-H). A very large difference might even lead to an ionic bond (e.g., NaCl).
-
Understanding Molecular Geometry: The polarity of individual bonds influences the overall molecular polarity and geometry. This, in turn, affects the physical and chemical properties of the molecule, such as boiling point, melting point, solubility, and reactivity.
-
Explaining Chemical Reactivity: Electronegativity plays a crucial role in predicting the reactivity of elements and compounds. Highly electronegative elements tend to be strong oxidizing agents (they readily gain electrons), while elements with low electronegativity act as strong reducing agents (they readily lose electrons).
-
Interpreting Spectroscopic Data: Electronegativity differences can be correlated with spectroscopic parameters like bond lengths and vibrational frequencies, providing insights into molecular structure and bonding.
-
Predicting Acid-Base Behavior: Electronegativity influences the acidity and basicity of compounds. For instance, the electronegativity of the central atom in an oxyacid affects its acid strength.
Electronegativity Scales
Various scales are used to quantify electronegativity. The most common are:
-
Pauling Scale: Developed by Linus Pauling, this scale is widely used and assigns fluorine (F) the highest electronegativity value of 4.0.
-
Mulliken Scale: This scale relates electronegativity to ionization energy and electron affinity.
-
Allred-Rochow Scale: This scale considers the effective nuclear charge and the atomic radius.
While these scales differ slightly in their absolute values, they all reveal the same general periodic trends.
Conclusion: Addressing the Initial Misconception
It is crucial to reiterate that the initial statement, "electronegativity increases as the size of the atom increases," is fundamentally incorrect. The correct relationship is an inverse one. Electronegativity generally decreases as atomic size increases due to the increasing distance between the nucleus and valence electrons, resulting in weaker attraction. Understanding this inverse relationship is fundamental for grasping the concepts of chemical bonding, reactivity, and the properties of compounds. The interplay of atomic size, effective nuclear charge, and other contributing factors provides a comprehensive picture of electronegativity's importance in chemistry. By appreciating these nuanced relationships, we can better understand and predict the behavior of matter at the atomic and molecular levels. Further research into specific elements and their unique electronic configurations can refine our understanding of these fundamental trends.
Latest Posts
Latest Posts
-
Energy From Moving Air Is Produced By
Apr 09, 2025
-
10 Out Of 16 As A Percentage
Apr 09, 2025
-
How Many Hours In 800 Minutes
Apr 09, 2025
-
How Many Inches In 8 Mm
Apr 09, 2025
-
How Much Is 90 Minutes In Hours
Apr 09, 2025
Related Post
Thank you for visiting our website which covers about Electronegativity Increases As The Size Of The Atom Increases . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.