How Are Hydrogens Removed From Polyprotic Acids
Kalali
Mar 23, 2025 · 6 min read
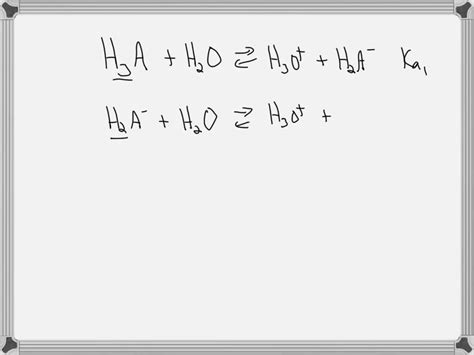
Table of Contents
How Are Hydrogens Removed from Polyprotic Acids? A Comprehensive Guide
Polyprotic acids, unlike monoprotic acids, possess multiple ionizable hydrogen atoms. This characteristic significantly influences their behavior in solution and how they interact with bases. Understanding how these hydrogens are removed is crucial in various chemical applications, from industrial processes to biological systems. This article will delve into the intricacies of hydrogen removal from polyprotic acids, exploring the underlying principles, influencing factors, and practical implications.
Understanding Polyprotic Acids
Before diving into the hydrogen removal process, let's establish a firm foundation by defining polyprotic acids and highlighting their distinguishing features.
Definition and Examples
A polyprotic acid is an acid that can donate more than one proton (H⁺) per molecule to a base. These acids undergo a stepwise ionization process, releasing protons one at a time. Common examples include:
- Sulfuric acid (H₂SO₄): A strong diprotic acid, readily donating two protons.
- Phosphoric acid (H₃PO₄): A weak triprotic acid, releasing three protons sequentially.
- Carbonic acid (H₂CO₃): A weak diprotic acid found in carbonated beverages and blood.
- Oxalic acid (C₂H₂O₄): A weak diprotic acid present in many plants.
- Citric acid (C₆H₈O₇): A weak triprotic acid found in citrus fruits.
Stepwise Ionization: The Key to Hydrogen Removal
The key to understanding hydrogen removal lies in the stepwise ionization process. Each ionization step has its own acid dissociation constant (Ka), reflecting the extent of proton donation at that stage. For example, phosphoric acid's ionization proceeds as follows:
- H₃PO₄ ⇌ H⁺ + H₂PO₄⁻ (Ka₁)
- H₂PO₄⁻ ⇌ H⁺ + HPO₄²⁻ (Ka₂)
- HPO₄²⁻ ⇌ H⁺ + PO₄³⁻ (Ka₃)
Notice that each step produces a conjugate base that can further donate a proton. The Ka values decrease progressively (Ka₁ > Ka₂ > Ka₃) because it becomes increasingly difficult to remove a proton from a negatively charged species.
Factors Influencing Hydrogen Removal
Several factors can significantly influence the rate and extent of hydrogen removal from polyprotic acids. These include:
Strength of the Acid
Strong polyprotic acids, like sulfuric acid, readily donate their protons. The first ionization is essentially complete, meaning almost all the initial acid molecules lose their first proton. The second ionization may be less complete, depending on the specific acid.
Weak polyprotic acids, like phosphoric acid, only partially ionize in solution. The equilibrium lies far to the left, meaning only a small fraction of the acid molecules lose their protons at each step.
Concentration of the Acid
A higher concentration of acid leads to a higher concentration of protons in solution. However, this doesn't necessarily mean that more protons will be removed from each individual acid molecule; it only influences the overall number of protons available.
pH of the Solution
The pH of the solution dictates the availability of hydroxide ions (OH⁻), which act as proton acceptors. A higher pH (more basic solution) favors proton removal, shifting the equilibrium towards the formation of conjugate bases. A lower pH (more acidic solution) hinders proton removal.
Temperature
Temperature affects the equilibrium constant (Ka) for each ionization step. Generally, increasing the temperature increases the Ka values, favoring proton removal. This is because higher temperatures provide the energy needed to break the bonds holding the protons.
Presence of Other Ions
The presence of other ions in the solution can influence the ionic strength and thus affect the activity of the acid and its conjugate bases. This can lead to changes in the equilibrium constants and the extent of ionization. For example, the presence of common ions (ions common to the acid or its conjugate bases) can suppress ionization according to the common ion effect.
Nature of the Base
The strength of the base used to remove the protons significantly affects the extent of ionization. Strong bases, like NaOH, readily accept protons, pushing the equilibrium towards the formation of conjugate bases, while weak bases lead to less complete ionization.
Methods for Removing Hydrogens
Several methods can be employed to remove hydrogens from polyprotic acids, each with its own advantages and disadvantages.
Neutralization Reactions
This is the most common method, involving reacting the polyprotic acid with a base. The number of base equivalents needed to completely neutralize the acid depends on the number of ionizable protons. For instance, complete neutralization of one mole of phosphoric acid requires three moles of a monobasic base like NaOH.
The reaction proceeds stepwise, with each proton being removed sequentially:
- H₃PO₄ + NaOH → H₂PO₄⁻ + Na⁺ + H₂O
- H₂PO₄⁻ + NaOH → HPO₄²⁻ + Na⁺ + H₂O
- HPO₄²⁻ + NaOH → PO₄³⁻ + Na⁺ + H₂O
Electrolysis
Electrolysis can be used to remove protons under specific conditions. By applying an electric current, protons can be oxidized at the anode, effectively removing them from the solution. This method is less commonly used for polyprotic acids due to the complexity of controlling the stepwise removal of protons and the potential for side reactions.
Chemical Reactions
Specific chemical reactions can be designed to selectively remove protons from polyprotic acids. These reactions often involve the use of specific reagents that react preferentially with certain protonated species. However, the design and implementation of such reactions are highly specific to the polyprotic acid in question and the desired outcome.
Applications of Polyprotic Acid Hydrogen Removal
The ability to control and manipulate the removal of hydrogens from polyprotic acids has significant implications across various fields:
Buffer Solutions
Polyprotic acids and their conjugate bases form excellent buffer solutions. By carefully selecting the appropriate ratio of acid and conjugate base, a specific pH can be maintained within a narrow range. This is crucial in biochemical applications, where maintaining a constant pH is essential for enzyme activity.
Industrial Processes
Polyprotic acids are extensively used in various industrial processes, including:
- Food industry: Citric acid acts as a flavoring agent, preservative, and chelating agent.
- Pharmaceutical industry: Polyprotic acids are used in drug formulations and as intermediates in drug synthesis.
- Water treatment: Polyprotic acids help control the pH and remove impurities from water.
- Fertilizers: Phosphoric acid is a key component of many fertilizers.
Biological Systems
Polyprotic acids play crucial roles in biological systems. For example, phosphoric acid is a key component of nucleotides and nucleic acids, while carbonic acid plays a vital role in maintaining blood pH. The controlled removal of protons is crucial for regulating biological processes.
Conclusion
The removal of hydrogens from polyprotic acids is a complex yet fundamental process governed by multiple factors. Understanding these factors and the available methods for proton removal is critical for various applications across different scientific and industrial domains. Whether it involves neutralizing an acid, creating a buffer solution, or understanding the intricacies of biological systems, mastering the manipulation of hydrogen removal in polyprotic acids is a key skill for chemists and scientists alike. Further research into this area continues to refine our understanding and uncover new applications for these versatile compounds.
Latest Posts
Latest Posts
-
104 Cm Is How Many Inches
Mar 25, 2025
-
Number Of Valence Electrons Of Potassium
Mar 25, 2025
-
How Many Feet Is 230 Cm
Mar 25, 2025
-
64 Ounces Is How Many Liters
Mar 25, 2025
-
What Is 6 As A Decimal
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about How Are Hydrogens Removed From Polyprotic Acids . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
