How Did He Know That The Nucleus Was Positively Charged
Kalali
Mar 19, 2025 · 6 min read
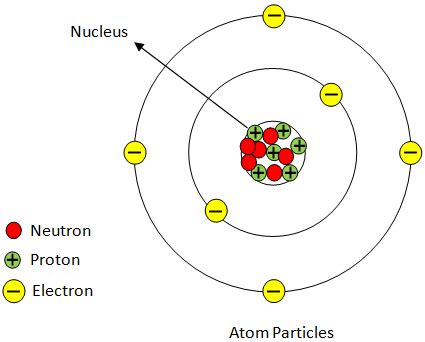
Table of Contents
How Did He Know That the Nucleus Was Positively Charged? Unraveling the Discovery of the Nucleus's Positive Charge
The discovery that the atomic nucleus carries a positive charge wasn't a "eureka!" moment from a single individual. Instead, it was a culmination of numerous experiments and brilliant deductions spanning several decades, building upon earlier atomic models and gradually refining our understanding. This journey involved several key players, notably Ernest Rutherford, whose gold foil experiment is most famously associated with this discovery, but also scientists whose preceding work laid the crucial groundwork. Understanding how the positive charge of the nucleus was determined requires exploring the scientific landscape of the late 19th and early 20th centuries.
The Precursors: Setting the Stage for Discovery
Before Rutherford's groundbreaking experiment, scientists had already accumulated significant data about atoms and their properties. Several crucial discoveries paved the way:
The Discovery of the Electron: J.J. Thomson's cathode ray experiments in the late 1890s demonstrated the existence of negatively charged particles, much smaller than atoms, which he termed "electrons." This discovery dramatically altered the prevailing model of the atom as an indivisible, fundamental unit. Thomson's "plum pudding" model proposed a positively charged sphere with negatively charged electrons embedded within it, like plums in a pudding.
Radioactivity: The discovery of radioactivity by Henri Becquerel in 1896 and subsequent research by Marie and Pierre Curie revealed that certain elements spontaneously emitted radiation. This radiation was later categorized into alpha, beta, and gamma rays, each with distinct properties. These discoveries hinted at a complex internal structure within the atom, far more intricate than Thomson's simple model suggested.
Understanding Alpha Particles: The alpha particles, identified as doubly ionized helium atoms (He²⁺), played a pivotal role in Rutherford's experiment. These particles, carrying a known positive charge and considerable mass, were used as projectiles to probe the internal structure of atoms. Their positive charge, combined with their relatively high mass, made them ideal for such investigations. Early studies of alpha particle scattering from thin metallic foils provided hints of a concentrated positive charge within the atom.
Rutherford's Gold Foil Experiment: The Pivotal Breakthrough
Ernest Rutherford, along with his team (including Hans Geiger and Ernest Marsden), conducted a series of experiments in 1909 that revolutionized our understanding of the atom. This famous gold foil experiment involved bombarding a thin gold foil with a beam of alpha particles. The experiment's setup included a radioactive source emitting alpha particles, the gold foil itself, a fluorescent screen to detect scattered particles, and a microscope to observe the scintillations (flashes of light) produced when alpha particles struck the screen.
The Unexpected Results: According to Thomson's plum pudding model, the alpha particles, being relatively massive and positively charged, should have passed through the gold foil with only minimal deflection. The expectation was a slight scattering, perhaps a minor deviation from a straight path. However, the results were astonishingly different.
The Astonishing Observations: The experiment revealed that:
- Most alpha particles passed straight through the gold foil: This observation supported the idea that the atom was mostly empty space.
- A small percentage of alpha particles were deflected at large angles: This was completely unexpected. The large deflections implied a powerful repulsive force acting on the alpha particles.
- A very small number of alpha particles were deflected back towards the source: This was the most striking result. It suggested that the alpha particles had encountered a highly concentrated positive charge, causing them to recoil directly back.
Interpreting the Results: Rutherford's genius lay in his ability to interpret these observations. He concluded that:
-
The atom is mostly empty space: The fact that most alpha particles passed through the foil unimpeded indicated that the positive charge wasn't uniformly distributed throughout the atom as Thomson proposed.
-
The atom contains a tiny, dense, positively charged nucleus: The large-angle deflections and backward scattering could only be explained by a concentrated positive charge at the atom's center, which Rutherford named the "nucleus." The repulsive force between the positively charged alpha particles and the nucleus caused the deflections.
-
The nucleus contains most of the atom's mass: The large deflections suggested that the nucleus contained most of the atom's mass, concentrated in a small volume. The electrons, being much lighter, occupy the vast majority of the atom's volume.
The Implications of Rutherford's Discovery
Rutherford's gold foil experiment and its interpretation had profound implications:
-
Overthrow of the Plum Pudding Model: Thomson's model was definitively refuted. The atom was not a uniformly charged sphere but rather a system with a tiny, dense, positively charged nucleus at its center and electrons orbiting it.
-
The Nuclear Model of the Atom: Rutherford proposed the nuclear model, which accurately depicted the atom as having a small, dense, positively charged nucleus at the center and electrons orbiting around it, mostly empty space. This model provided a framework for future research on atomic structure.
-
Foundation for Future Discoveries: This discovery laid the groundwork for further research, including the development of the Bohr model, which incorporated quantum theory to explain the stability of the atom, and the subsequent discoveries of protons and neutrons within the nucleus.
Further Refinements and the Role of Other Scientists
While Rutherford's experiment established the existence of a positively charged nucleus, the precise nature of the positive charge and its composition remained to be discovered.
-
The Discovery of the Proton: Later experiments revealed the existence of the proton, a positively charged particle found within the nucleus. Ernest Rutherford himself played a significant role in identifying the proton.
-
The Discovery of the Neutron: James Chadwick's discovery of the neutron in 1932 completed the picture of the atomic nucleus. Neutrons, which are electrically neutral particles, were found to be another constituent of the nucleus.
The Charge-to-Mass Ratio: Experiments to determine the charge-to-mass ratio of alpha particles, and other charged particles emanating from radioactive decay, were crucial in establishing the positive nature and magnitude of the charge associated with the nucleus. These ratios, when combined with measurements of the scattering angles in experiments like Rutherford's, helped solidify the understanding of the nucleus’s positive charge.
Conclusion: A Journey of Scientific Discovery
The discovery of the positively charged nucleus wasn't a sudden revelation but rather a process of incremental progress built upon earlier scientific breakthroughs and ingeniously designed experiments. Rutherford's gold foil experiment, however, stands out as a pivotal moment, definitively establishing the presence of a tiny, dense, positively charged center within the atom. His interpretation of the experimental results, along with the subsequent discoveries of protons and neutrons, ultimately provided a comprehensive understanding of the structure and charge of the atomic nucleus. This journey showcases the collaborative nature of scientific progress, where numerous scientists, building upon each other's work, gradually unravel the mysteries of the natural world. The story of the nucleus's positive charge serves as a testament to the power of experimental investigation, innovative thinking, and the relentless pursuit of knowledge.
Latest Posts
Latest Posts
-
Is Frying An Egg A Physical Change
Mar 19, 2025
-
What Is The Role Of Producers In An Ecosystem
Mar 19, 2025
-
Is Sour Taste A Physical Property Or Chemical Property
Mar 19, 2025
-
11 Out Of 15 Is What Percentage
Mar 19, 2025
-
Mistletoe And Spruce Tree Symbiotic Relationship
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about How Did He Know That The Nucleus Was Positively Charged . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
