How Does Longer Contact Increase Dissolution
Kalali
Mar 25, 2025 · 6 min read
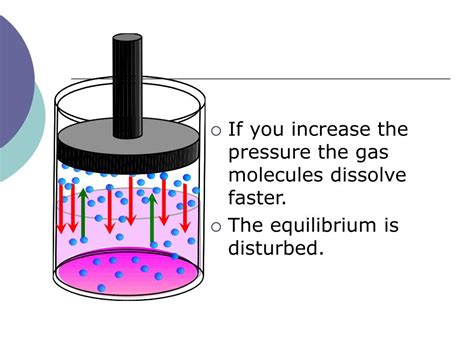
Table of Contents
How Does Longer Contact Time Increase Dissolution?
Dissolution, the process by which a solid substance dissolves into a solvent to form a solution, is a fundamental phenomenon in numerous scientific and industrial fields. Understanding the factors that influence dissolution rate is crucial for optimizing various processes, from drug delivery to chemical manufacturing. One of the most significant factors affecting dissolution is contact time, the duration for which the solid is in contact with the solvent. This article delves into the intricate relationship between longer contact time and increased dissolution, exploring the underlying mechanisms and practical implications.
The Fundamentals of Dissolution
Before exploring the impact of contact time, it's essential to understand the basic principles governing dissolution. Dissolution is a kinetic process, meaning its rate depends on several factors:
- Solubility: The inherent ability of a substance to dissolve in a particular solvent. Highly soluble substances dissolve faster than poorly soluble ones.
- Surface Area: A larger surface area of the solid exposed to the solvent leads to faster dissolution, as more solute molecules can interact simultaneously with the solvent. This is why powdered substances dissolve quicker than large chunks.
- Temperature: Higher temperatures generally increase the kinetic energy of molecules, leading to faster dissolution. Increased molecular motion facilitates interactions between solute and solvent.
- Agitation: Stirring or shaking the solution enhances the rate of dissolution by constantly replenishing the solvent layer near the solid's surface. This prevents the formation of a saturated layer that impedes further dissolution.
- Solvent Properties: The nature of the solvent, such as its polarity and viscosity, influences its ability to dissolve the solute. Polar solvents generally dissolve polar solutes, and vice versa. Viscosity affects the rate of diffusion of solute molecules away from the solid surface.
The Role of Contact Time in Dissolution
Contact time plays a pivotal role in the dissolution process. The longer the solid is in contact with the solvent, the greater the extent of dissolution. This seemingly simple statement underpins several complex mechanisms:
1. Saturation and Equilibrium
Dissolution is not an infinitely rapid process. Initially, the rate of dissolution is relatively high. However, as solute molecules dissolve into the solvent, the concentration of the solute in the solvent increases. This gradually leads to a decrease in the dissolution rate.
Eventually, a state of saturation is reached where the rate of dissolution equals the rate of precipitation (the solute coming out of the solution). At this point, the solution is saturated, and no further net dissolution occurs. Equilibrium is achieved, and the concentration of the solute remains constant.
Longer contact time allows the system to approach this saturation point more closely. While complete dissolution might not always be achieved, a longer contact period results in a higher concentration of dissolved solute.
2. Diffusion Limitations
The rate of dissolution can be limited by the diffusion of solute molecules away from the solid surface. A layer of saturated solution forms around the solid, creating a concentration gradient. Solute molecules must diffuse through this layer to reach the bulk solvent.
Extended contact time allows for more efficient diffusion. The gradual dispersal of dissolved solute molecules from the solid surface maintains a concentration gradient, promoting continuous dissolution. This is especially relevant for sparingly soluble substances where diffusion limitations are more pronounced.
3. Chemical Reactions and Complexation
In some cases, dissolution may involve chemical reactions between the solute and the solvent, or the formation of complexes. These reactions can be slow and require sufficient contact time to reach completion.
For instance, certain metal oxides may require prolonged exposure to an acidic solution to undergo complete dissolution. The reaction between the metal oxide and acid molecules needs sufficient time to proceed to completion. Similarly, the formation of soluble complexes between metal ions and ligands often requires extended contact time for the equilibrium to be reached.
4. Solid State Transformations
Some solids might undergo structural changes during the dissolution process, such as particle size reduction due to attrition or the transformation of crystalline forms. These transformations can influence the dissolution rate, and sufficient contact time is necessary for these changes to occur. For example, a polymorphic form of a drug with lower solubility might transform into a more soluble form over time, leading to increased dissolution with extended contact.
Practical Implications of Extended Contact Time
The impact of longer contact time on dissolution has significant practical implications across diverse fields:
1. Pharmaceutical Industry
In drug delivery, the dissolution rate of a drug substance is crucial for its bioavailability. Extended contact time ensures that a sufficient amount of the drug dissolves in the gastrointestinal tract to achieve the desired therapeutic effect. Formulation scientists carefully design drug products to optimize dissolution, considering factors such as particle size, excipients, and the physiological environment.
2. Chemical Engineering
In chemical processes, controlling dissolution is essential for achieving desired reaction rates and product yields. Longer contact times can be utilized to ensure complete dissolution of reactants before initiating a reaction, enhancing the efficiency and reproducibility of the process.
3. Environmental Science
The dissolution of pollutants in soil or water is a critical factor in environmental remediation. The contact time between pollutants and the surrounding medium determines the rate at which they dissolve and potentially migrate to other locations. Understanding the kinetics of pollutant dissolution allows for better strategies for environmental cleanup.
4. Food Science
The dissolution of food components, such as sugars and salts, influences taste, texture, and the overall sensory experience. The time it takes for these components to dissolve in the mouth contributes to the perception of flavor and mouthfeel.
5. Material Science
The dissolution of materials is crucial in various materials processing techniques. For example, etching processes rely on controlled dissolution to create specific surface features or remove unwanted material. The duration of etching is critical for achieving desired results.
Optimizing Contact Time for Enhanced Dissolution
While longer contact time generally increases dissolution, it's not always the most efficient approach. Other factors, such as surface area, agitation, and temperature, can significantly enhance dissolution. Optimal strategies involve a combination of these factors:
- Particle size reduction: Decreasing the particle size of the solid drastically increases the surface area exposed to the solvent, leading to faster dissolution.
- Agitation: Continuous mixing ensures that fresh solvent constantly interacts with the solid, preventing the formation of a saturated layer.
- Temperature control: Increasing the temperature can enhance the solubility and kinetic energy of molecules, leading to faster dissolution.
- Solvent selection: Using a solvent with high solubility for the solute is crucial. Moreover, the solvent's viscosity affects the diffusion rate of solute molecules.
Conclusion
Longer contact time undeniably plays a crucial role in increasing dissolution. It allows the system to approach saturation, improves diffusion, facilitates chemical reactions, and permits solid-state transformations, leading to a higher concentration of dissolved solute. However, optimizing dissolution is not solely dependent on contact time. A comprehensive approach involving particle size reduction, agitation, temperature control, and appropriate solvent selection ensures efficient and effective dissolution processes. Understanding these intricacies allows for precise control over dissolution in various applications, ultimately improving efficiency, performance, and outcomes across diverse scientific and industrial fields.
Latest Posts
Latest Posts
-
How Do Peppered Moths Spend The Winter
Mar 26, 2025
-
22 Feet Is How Many Meters
Mar 26, 2025
-
What Is 10 Percent Of 300
Mar 26, 2025
-
How Many Cm Is 57 Inches
Mar 26, 2025
-
What Is The Percentage Of 17 20
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about How Does Longer Contact Increase Dissolution . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
