How Is Baking A Cake A Chemical Change
Kalali
Mar 18, 2025 · 6 min read
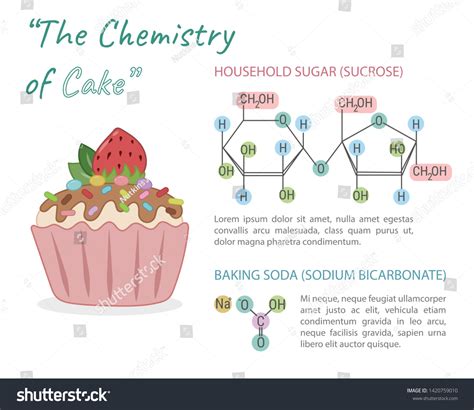
Table of Contents
How Baking a Cake is a Chemical Change: A Delicious Dive into Chemistry
Baking a cake is more than just a culinary art; it's a fascinating demonstration of chemical reactions. While the process might seem simple – mixing ingredients, baking, and enjoying – a deeper look reveals a complex interplay of chemical changes that transform humble ingredients into a fluffy, delicious treat. This article will explore the chemical reactions at play, explaining why baking a cake is undeniably a chemical change, not just a physical one.
Understanding Chemical vs. Physical Changes
Before delving into the cake-making chemistry, it's essential to clarify the difference between chemical and physical changes. A physical change alters the form or appearance of a substance but doesn't change its chemical composition. Think of melting ice – it changes from solid to liquid, but it remains H₂O. Conversely, a chemical change, also known as a chemical reaction, involves the rearrangement of atoms and molecules, forming new substances with different properties. This often involves breaking and forming chemical bonds. Burning wood, for example, is a chemical change, transforming wood into ash and gases.
The Chemistry of Cake Baking: A Step-by-Step Breakdown
The magic of cake baking lies in the series of chemical reactions triggered by combining and heating various ingredients. Let's examine these reactions step-by-step:
1. The Role of Flour: Gluten Development and Starch Gelatinization
Flour, primarily composed of starch and protein (gluten), undergoes significant changes during baking. When flour is mixed with water, the gluten proteins, gliadin, and glutenin, hydrate and form gluten. This gluten network is crucial for the cake's structure, providing elasticity and strength. The extent of gluten development depends on factors like the type of flour, mixing technique, and resting time. Over-mixing can lead to tough gluten, while under-mixing results in a weak structure.
Simultaneously, the starch granules in the flour absorb water and swell. This process, known as starch gelatinization, contributes to the cake's texture and moisture retention. Heat further strengthens this gelatinized starch network, providing additional support to the cake structure.
2. Leavening Agents: The Rise of the Cake
Leavening agents are crucial for the cake's rise, creating a light and airy texture. Common leavening agents include baking powder, baking soda, and yeast (though less common in cakes).
-
Baking powder: This is a combination of an acid (e.g., cream of tartar) and a base (e.g., sodium bicarbonate). When mixed with water, the acid and base react, producing carbon dioxide gas. Heat accelerates this reaction, causing the cake batter to rise. It's a double-acting leavening agent, releasing some gas upon mixing and the rest during baking.
-
Baking soda: This is a base (sodium bicarbonate) that requires an acidic ingredient in the recipe to react and produce carbon dioxide. The acid can come from ingredients like buttermilk, lemon juice, or brown sugar. Baking soda is a single-acting leavening agent, releasing gas primarily upon mixing.
The carbon dioxide gas produced by these reactions gets trapped within the gluten and starch network, creating the characteristic airy texture of a cake. This gas production is a clear indicator of a chemical change.
3. Sugar's Role: Sweetness, Browning, and Moisture Retention
Sugar contributes more than just sweetness to a cake. It plays a critical role in several chemical processes:
-
Browning: Sugar undergoes caramelization at high temperatures, resulting in the characteristic brown crust of the cake. This is a complex chemical reaction involving the breakdown of sugar molecules and the formation of various compounds responsible for the rich color and flavor.
-
Moisture retention: Sugar helps to retain moisture in the cake, preventing it from drying out. It competes with the starch for water molecules, ensuring a softer, moister crumb.
-
Tenderizing: Sugar interferes with gluten development, making the cake more tender. This is a physical interaction, but it affects the final product's texture in a significant way.
4. Eggs: Emulsification, Structure, and Leavening
Eggs are multi-tasking ingredients in cake baking. They contribute to the cake's structure, moisture, and richness through several mechanisms:
-
Emulsification: Egg yolks contain lecithin, an emulsifier that helps combine fats (like butter or oil) and water, preventing separation and creating a smooth batter. This is a crucial physical process that impacts the final texture.
-
Structure: Egg proteins denature (unfold) and coagulate (set) during baking, contributing to the cake's structure. This structural change is a chemical reaction that solidifies the batter.
-
Leavening: Egg whites contain air bubbles which expand during baking, providing additional leavening. This is aided by the heat-induced protein denaturation.
5. Fats: Tenderness, Moisture, and Flavor
Fats, such as butter or oil, contribute to the cake's tenderness, moisture, and flavor. They coat the gluten strands, preventing excessive gluten development, resulting in a softer cake. The fats also contribute to the cake's richness and flavor. While fat melting is a physical change, its interaction with other ingredients triggers chemical changes affecting the final structure and texture.
6. The Maillard Reaction: Flavor and Color Development
As the cake bakes, a significant chemical reaction occurs between the amino acids in proteins (from flour and eggs) and reducing sugars (from sugar). This is the Maillard reaction, responsible for the characteristic brown crust and the complex, savory flavor notes in the cake. This reaction requires high temperatures and is a key contributor to the appealing aroma and taste of baked goods.
Irreversible Changes: The Hallmark of a Chemical Reaction
One of the most compelling arguments for cake baking being a chemical change is the irreversibility of the process. You can't easily reverse the changes that occur during baking. You can't simply un-bake a cake to return the ingredients to their original states. The formation of new compounds through reactions like the Maillard reaction and the setting of egg proteins are permanent alterations, confirming the chemical nature of the baking process.
Conclusion: A Delicious Symphony of Chemical Reactions
Baking a cake is a delightful journey into the world of chemistry. The seemingly simple process is actually a complex orchestration of various chemical reactions, transforming raw ingredients into a delectable final product. From the development of the gluten network to the Maillard reaction, each stage involves the formation of new substances with different properties. The irreversability of these changes and the formation of new compounds undeniably classify cake baking as a chemical change. Understanding these underlying chemical processes allows bakers to better control the baking process, achieving the desired texture, flavor, and appearance in their creations. So, next time you bake a cake, remember that you're not just following a recipe; you're conducting a fascinating chemical experiment!
Latest Posts
Latest Posts
-
High Frequency Wave Vs Low Frequency Wave
Mar 19, 2025
-
Temperate Deciduous Forest Oak Tree Adaptations
Mar 19, 2025
-
What Animals Can See Human Bioluminescence
Mar 19, 2025
-
Is Sour Taste A Physical Property
Mar 19, 2025
-
How Many Kilos Are 20 Pounds
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about How Is Baking A Cake A Chemical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
