How Many Atoms Are In H2o2
Kalali
Mar 28, 2025 · 5 min read
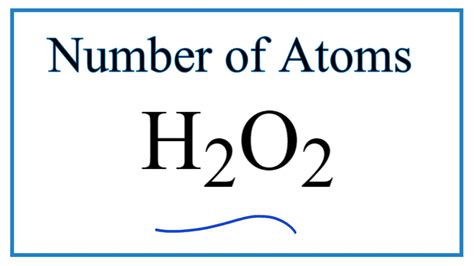
Table of Contents
How Many Atoms Are in H₂O₂? A Deep Dive into Molecular Composition and Counting
Determining the number of atoms in a molecule like hydrogen peroxide (H₂O₂) might seem straightforward, but it opens the door to understanding fundamental concepts in chemistry, including molecular formulas, molar mass, and Avogadro's number. This article will not only answer the titular question but also explore the broader implications of atomic counting within molecules and delve into related calculations.
Understanding the Chemical Formula: H₂O₂
The chemical formula H₂O₂ represents hydrogen peroxide. This formula provides a concise description of the molecule's composition:
- H: Represents a hydrogen atom.
- 2: Indicates there are two hydrogen atoms present in the molecule.
- O: Represents an oxygen atom.
- 2: Indicates there are two oxygen atoms present in the molecule.
Therefore, a single molecule of hydrogen peroxide contains two hydrogen atoms and two oxygen atoms. This brings the total number of atoms in one H₂O₂ molecule to four.
Beyond the Single Molecule: Scaling Up to Moles
While knowing the number of atoms in a single molecule is useful, it's often more practical to work with larger quantities of substances. This is where the concept of the mole comes in. A mole is a unit of measurement in chemistry that represents Avogadro's number (approximately 6.022 x 10²³) of entities. These entities can be atoms, molecules, ions, or any other specified unit.
Avogadro's Number: The Cornerstone of Chemical Calculations
Avogadro's number is crucial for bridging the gap between the microscopic world of atoms and molecules and the macroscopic world of laboratory measurements. It allows chemists to relate the number of particles to the mass of a substance.
Calculating Atoms in a Mole of H₂O₂
Let's calculate the number of atoms in one mole of H₂O₂:
-
Atoms per molecule: As established earlier, each H₂O₂ molecule contains 4 atoms (2 hydrogen + 2 oxygen).
-
Molecules per mole: One mole of any substance contains Avogadro's number of molecules (6.022 x 10²³).
-
Total atoms: To find the total number of atoms in one mole of H₂O₂, multiply the number of atoms per molecule by the number of molecules per mole:
4 atoms/molecule * 6.022 x 10²³ molecules/mol = 2.409 x 10²⁴ atoms/mol
Therefore, one mole of hydrogen peroxide contains approximately 2.409 x 10²⁴ atoms.
Molar Mass and Atomic Mass: Connecting Mass and Number of Atoms
The molar mass of a substance is the mass of one mole of that substance, expressed in grams per mole (g/mol). It's directly related to the atomic masses of the constituent elements.
Calculating the Molar Mass of H₂O₂
To calculate the molar mass of H₂O₂, we need the atomic masses of hydrogen and oxygen:
- Hydrogen (H): Approximately 1.008 g/mol
- Oxygen (O): Approximately 16.00 g/mol
Molar mass of H₂O₂ = (2 * atomic mass of H) + (2 * atomic mass of O) = (2 * 1.008 g/mol) + (2 * 16.00 g/mol) = 34.016 g/mol
This means that one mole of hydrogen peroxide weighs approximately 34.016 grams.
Relating Molar Mass and Avogadro's Number
The molar mass links the mass of a substance to the number of molecules (and therefore atoms) present. For instance, 34.016 grams of H₂O₂ contains 6.022 x 10²³ molecules, which in turn contain 2.409 x 10²⁴ atoms.
Practical Applications and Further Explorations
The ability to calculate the number of atoms in a molecule has wide-ranging applications across various fields:
- Stoichiometry: This area of chemistry uses mole calculations to determine the quantities of reactants and products in chemical reactions. Knowing the number of atoms is fundamental for balancing equations and predicting yields.
- Analytical Chemistry: Techniques like titration and spectroscopy rely on understanding the relationships between mass, moles, and the number of atoms or molecules to analyze the composition of samples.
- Material Science: The atomic composition of materials directly influences their properties. Calculating the number of atoms helps in designing and characterizing new materials with specific characteristics.
- Biochemistry: Understanding the composition of biological molecules, such as proteins and DNA, requires knowledge of the number and types of atoms involved.
Beyond H₂O₂: Extending the Concept to Other Molecules
The principles discussed here can be applied to any molecule. To calculate the number of atoms in any compound:
- Determine the chemical formula: This gives the number of each type of atom in one molecule.
- Calculate the total number of atoms per molecule: Add up the number of atoms of each element.
- Multiply by Avogadro's number: For one mole of the substance, multiply the total atoms per molecule by 6.022 x 10²³.
Conclusion: The Significance of Atomic Counting
Understanding how to determine the number of atoms in a molecule like H₂O₂ is more than just a simple calculation. It's a fundamental skill that underpins much of chemistry and related fields. By grasping the concepts of molecular formulas, Avogadro's number, and molar mass, we gain a powerful tool for analyzing and predicting the behavior of matter at both the microscopic and macroscopic levels. The seemingly simple question of "how many atoms are in H₂O₂?" opens a world of possibilities in understanding the building blocks of our universe. From stoichiometric calculations to material science breakthroughs, the ability to quantify atoms is a cornerstone of scientific advancement. This article serves as a solid foundation for exploring more complex chemical concepts and expanding your knowledge of the fascinating world of atoms and molecules.
Latest Posts
Latest Posts
-
What Is The Boiling Point Of Saltwater
Mar 31, 2025
-
What Are The 2 Parts Of A Solution
Mar 31, 2025
-
What Are The Chemical Equations Of Photosynthesis And Cellular Respiration
Mar 31, 2025
-
What Is 57 Celsius In Fahrenheit
Mar 31, 2025
-
How Much Is 42 Inches In Feet
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about How Many Atoms Are In H2o2 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
