How Many Atoms Are In Hydrogen Peroxide
Kalali
Mar 19, 2025 · 5 min read
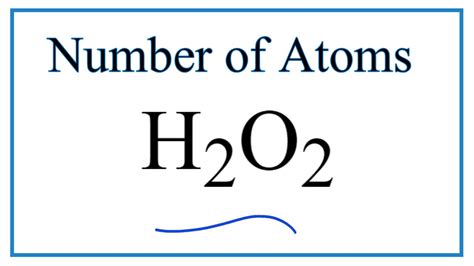
Table of Contents
How Many Atoms are in Hydrogen Peroxide? A Deep Dive into Molecular Composition
Hydrogen peroxide, a common household antiseptic and bleaching agent, is a simple yet fascinating chemical compound. Its molecular formula, H₂O₂, reveals its fundamental composition: two hydrogen atoms and two oxygen atoms. However, understanding the sheer number of atoms present in even a tiny amount of hydrogen peroxide requires delving into the world of moles, Avogadro's number, and the fundamental principles of chemistry. This article will explore this topic in depth, providing a clear understanding of how to calculate the number of atoms in hydrogen peroxide, along with related concepts and applications.
Understanding the Molecular Structure of Hydrogen Peroxide
Before we delve into the calculations, let's solidify our understanding of hydrogen peroxide's molecular structure. The formula H₂O₂ tells us that each molecule of hydrogen peroxide contains:
- Two hydrogen atoms (H): These are the smallest and lightest atoms, each possessing one proton and one electron.
- Two oxygen atoms (O): These atoms are significantly larger and heavier than hydrogen, each containing eight protons, eight neutrons (in the most common isotope), and eight electrons.
The atoms are bonded together covalently, meaning they share electrons to form stable molecules. The oxygen atoms are connected by a single covalent bond, and each oxygen atom is also bonded to a hydrogen atom. This arrangement gives hydrogen peroxide its unique chemical properties.
The Mole: The Chemist's Counting Unit
To determine the number of atoms in a macroscopic sample of hydrogen peroxide, we need a unit that can bridge the gap between the microscopic world of atoms and molecules and the macroscopic world of grams and liters. This unit is the mole (mol). One mole is defined as the amount of substance containing Avogadro's number (approximately 6.022 x 10²³) of elementary entities (atoms, molecules, ions, etc.).
Avogadro's number is a fundamental constant in chemistry, representing the number of atoms in 12 grams of carbon-12. It’s a colossal number, highlighting the incredibly tiny scale of atoms and molecules.
Calculating the Molar Mass of Hydrogen Peroxide
To work with moles, we need to determine the molar mass of hydrogen peroxide. This is the mass of one mole of H₂O₂ molecules. We can calculate it using the atomic masses of hydrogen and oxygen:
- Atomic mass of hydrogen (H): Approximately 1.008 g/mol
- Atomic mass of oxygen (O): Approximately 15.999 g/mol
Therefore, the molar mass of H₂O₂ is:
(2 x 1.008 g/mol) + (2 x 15.999 g/mol) = 34.014 g/mol
This means that one mole of hydrogen peroxide weighs approximately 34.014 grams.
Connecting Moles, Atoms, and Avogadro's Number
Now, we can link moles, Avogadro's number, and the number of atoms. One mole of hydrogen peroxide (H₂O₂) contains Avogadro's number (6.022 x 10²³) of H₂O₂ molecules. Since each molecule contains four atoms (two hydrogen and two oxygen), one mole of hydrogen peroxide contains:
4 atoms/molecule x 6.022 x 10²³ molecules/mol = 2.409 x 10²⁴ atoms/mol
This calculation shows that one mole of hydrogen peroxide contains a staggering 2.409 x 10²⁴ atoms.
Calculating the Number of Atoms in a Specific Mass of Hydrogen Peroxide
Let's apply this knowledge to calculate the number of atoms in a specific mass of hydrogen peroxide. Suppose we have 10 grams of hydrogen peroxide. First, we need to determine the number of moles:
Number of moles = mass (g) / molar mass (g/mol) = 10 g / 34.014 g/mol ≈ 0.294 moles
Now, we can calculate the number of molecules:
Number of molecules = number of moles x Avogadro's number = 0.294 mol x 6.022 x 10²³ molecules/mol ≈ 1.77 x 10²³ molecules
Finally, we can calculate the total number of atoms:
Number of atoms = number of molecules x 4 atoms/molecule ≈ 7.08 x 10²³ atoms
Therefore, 10 grams of hydrogen peroxide contains approximately 7.08 x 10²³ atoms.
Applications and Importance of Understanding Atom Count
Understanding how to calculate the number of atoms in a substance like hydrogen peroxide is crucial in various fields:
1. Chemistry and Chemical Reactions:
Accurate calculations of atom numbers are essential for balancing chemical equations, determining reaction yields, and understanding stoichiometry. This is vital in various chemical processes, from industrial production to laboratory experiments.
2. Material Science:
In material science, understanding the atomic composition of materials is essential for tailoring their properties. This knowledge is vital in developing new materials with specific characteristics.
3. Pharmaceutical Sciences:
Accurate calculations are crucial in pharmaceutical research and development. Determining the precise number of atoms in a drug molecule is essential for understanding its properties and dosage calculations.
4. Environmental Science:
Understanding the atomic composition of pollutants is crucial in environmental monitoring and remediation efforts. This knowledge helps in assessing environmental impact and developing solutions.
Conclusion: A Microscopic World with Macroscopic Implications
While the number of atoms in a seemingly small amount of hydrogen peroxide may seem incomprehensible, this calculation demonstrates the immense scale involved in dealing with atoms and molecules. The concepts of moles and Avogadro's number provide a crucial bridge between the microscopic and macroscopic worlds, allowing chemists and scientists to quantify and work with vast numbers of atoms and molecules. Understanding these fundamental concepts is crucial for advancements in various scientific and technological fields. The ability to accurately calculate the number of atoms in a given mass of a substance like hydrogen peroxide, therefore, is a cornerstone of modern science and its applications. From understanding chemical reactions to developing new materials and medicines, this knowledge plays a vital role in shaping our world.
Latest Posts
Latest Posts
-
Does Gold Show Up On A Metal Detector
Mar 19, 2025
-
In Fully Contracted Muscles The Actin Filaments Lie Side By Side
Mar 19, 2025
-
How Many Calories Are In 1g Of Uranium
Mar 19, 2025
-
10 Of 100 Is How Much
Mar 19, 2025
-
How Many Meters Is 150 Ft
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about How Many Atoms Are In Hydrogen Peroxide . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
