How Many Bonds Does Silicon Form
Kalali
Mar 22, 2025 · 5 min read
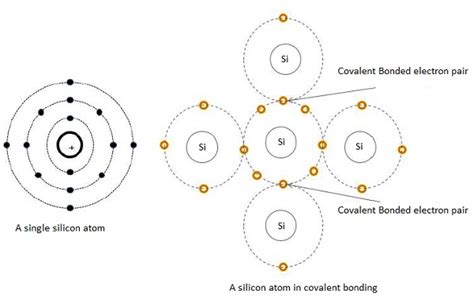
Table of Contents
How Many Bonds Does Silicon Form? Unveiling the Bonding Behavior of Silicon
Silicon, the heart of the microelectronics revolution, is a fascinating element whose properties stem directly from its bonding behavior. While often compared to carbon, its ability to form bonds differs in crucial ways, influencing its diverse applications in everything from computer chips to solar cells. So, how many bonds does silicon form? The simple answer is four, but the story behind this seemingly straightforward answer is far richer and more nuanced.
Understanding Silicon's Bonding Capacity
Silicon, like carbon, is a group 14 element, meaning it possesses four valence electrons in its outermost shell. These valence electrons are the key players in chemical bonding. Atoms strive for stability, often achieved by filling their outermost shell with eight electrons (the octet rule). Silicon achieves this stability by sharing its four valence electrons with other atoms through covalent bonds.
Covalent Bonding: The Foundation of Silicon's Structure
Covalent bonds are formed when two atoms share electrons to complete their outermost shells. In silicon's case, each silicon atom forms four covalent bonds with four neighboring silicon atoms, creating a strong and stable three-dimensional network. This tetrahedral arrangement is the fundamental building block of the silicon crystal structure.
The Significance of Four Bonds
The fact that silicon consistently forms four bonds is pivotal to understanding its properties:
- Crystalline Structure: The four covalent bonds result in a strong, rigid, and highly ordered crystal structure. This structure is responsible for silicon's hardness and high melting point.
- Semiconductor Properties: The energy gap between the valence band (where electrons are normally found) and the conduction band (where electrons can move freely) in silicon is just right for its semiconductor behavior. This allows silicon to conduct electricity under specific conditions, making it ideal for electronic components. The precise number of bonds and their arrangement contribute directly to this energy gap.
- Chemical Reactivity: While silicon forms four bonds readily, it's less reactive than carbon. This lower reactivity is partially attributed to the larger size of the silicon atom, resulting in weaker covalent bonds compared to carbon. This characteristic is crucial in its applications where stability is paramount.
Exceptions and Nuances in Silicon Bonding
While four bonds are the norm, there are instances where silicon's bonding behavior exhibits variations:
Silicon Hydrides (Silanes)
Silanes are compounds containing silicon and hydrogen. The simplest silane is monosilane (SiH₄), where silicon forms four bonds with four hydrogen atoms. More complex silanes exist with various silicon-silicon and silicon-hydrogen bonds, illustrating the versatility of silicon's bonding capacity within these specific chemical contexts.
Silicon Oxides (Silicates)
Silicon readily bonds with oxygen, forming a vast array of silicate compounds. These compounds form the backbone of many minerals and geological formations. While silicon typically forms four bonds, the oxygen atoms often bridge between multiple silicon atoms, leading to complex network structures. The bonding in silicates often involves a combination of covalent and ionic character, further diversifying the bonding picture.
Organosilicon Compounds
Organosilicon compounds involve silicon bonded to carbon atoms. These compounds are incredibly diverse and have found applications in diverse fields, including polymers, lubricants, and sealants. Similar to silicates, the bonding here involves a mix of covalent interactions, and the overall structure is determined by the nature of the organic groups attached to the silicon atoms.
Silicon Halides
Silicon also forms bonds with halogens (fluorine, chlorine, bromine, and iodine) to create silicon halides. These compounds showcase silicon's ability to bond with a wide range of electronegative atoms, further highlighting its bonding flexibility. The number of bonds remains consistent at four, but the properties of the silicon halides vary significantly depending on the halogen involved.
Factors Influencing Silicon's Bonding
Several factors influence the way silicon forms bonds:
Electronegativity
Electronegativity measures an atom's ability to attract electrons in a chemical bond. While silicon is less electronegative than oxygen or halogens, this difference in electronegativity impacts the polarity of the bonds it forms. Bonds with more electronegative atoms will have a partial ionic character.
Steric Hindrance
Steric hindrance refers to the spatial arrangement of atoms and how it can affect bond formation. Bulky groups attached to silicon can impede the formation of all four bonds, potentially leading to less than four bonds in certain cases.
Bond Energy
The strength of silicon's covalent bonds influences its chemical reactivity and stability. The energy required to break a silicon-silicon bond, for example, is significantly lower than the energy required to break a carbon-carbon bond, explaining the difference in reactivity between the two elements.
Applications Driven by Silicon's Bonding
Silicon's ability to consistently form four covalent bonds is the cornerstone of its countless applications:
- Microelectronics: The precise control over silicon's bonding allows for the creation of intricate microchips and transistors, forming the basis of modern electronics. The semiconductor properties arising from its bonding arrangement are key here.
- Solar Cells: Silicon's ability to absorb sunlight and convert it into electricity is directly related to its electronic structure, which is a product of its bonding arrangement.
- Ceramics and Glasses: Silicon dioxide (SiO₂) forms the basis for many ceramics and glasses, thanks to the strong and stable network structure arising from silicon's four bonds with oxygen.
- Polymers and Sealants: Organosilicon polymers, such as silicones, exhibit unique properties such as flexibility, heat resistance, and water repellency, again attributable to the underlying silicon-carbon and silicon-oxygen bonding.
Conclusion: A Foundation in Four Bonds
In summary, silicon predominantly forms four covalent bonds. This seemingly simple fact underpins the remarkable properties and vast applications of this element. Understanding the nuances of silicon's bonding—the interplay of electronegativity, steric hindrance, and bond energies—provides deeper insights into its behavior in various chemical contexts. Its consistent tetrahedral bonding arrangement shapes the crystalline structure, dictates its semiconductor properties, and ultimately drives its widespread use in countless technologies that shape our modern world. From the intricate circuits in our smartphones to the durable materials in our buildings, silicon's four bonds are a testament to the power of fundamental chemical principles in shaping our technological landscape.
Latest Posts
Latest Posts
-
What Percent Is 1 Out Of 5
Mar 23, 2025
-
Multi Step Equations Using Distributive Property
Mar 23, 2025
-
How Many Ounces In 1 Kilogram
Mar 23, 2025
-
What Percent Of 30 Is 90
Mar 23, 2025
-
Gravitational Force Of Sun On Earth
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about How Many Bonds Does Silicon Form . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
