How Many Electrons Are In The Outer Shell Of Carbon
Kalali
Mar 31, 2025 · 7 min read
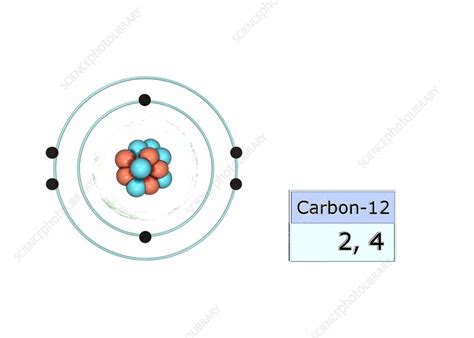
Table of Contents
How Many Electrons Are in the Outer Shell of Carbon? Understanding Carbon's Valence Electrons
Carbon, the cornerstone of organic chemistry and the basis of all known life, possesses a unique electronic structure that dictates its remarkable bonding capabilities. A fundamental aspect of understanding carbon's behavior lies in comprehending its electron configuration, specifically the number of electrons residing in its outermost shell. This article will delve into the details of carbon's electronic structure, explaining why the number of valence electrons is crucial for its reactivity and its role in forming diverse molecules. We'll explore the concept of valence electrons, delve into the periodic table's role in predicting electron configurations, and examine the implications of carbon's four valence electrons in the formation of organic compounds.
Understanding Electron Shells and Subshells
Before focusing on carbon, let's establish a basic understanding of electron shells and subshells. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons orbiting in distinct energy levels called shells. These shells are further divided into subshells, designated as s, p, d, and f, each capable of holding a specific number of electrons.
- Shell 1 (K shell): Holds a maximum of 2 electrons, all in the 1s subshell.
- Shell 2 (L shell): Holds a maximum of 8 electrons, distributed across the 2s (2 electrons) and 2p (6 electrons) subshells.
- Shell 3 (M shell): Holds a maximum of 18 electrons, distributed across the 3s, 3p, and 3d subshells.
- Shell 4 (N shell) and beyond: The pattern continues, with increasing electron capacity in each subsequent shell.
The arrangement of electrons in these shells and subshells is crucial in determining an atom's chemical properties and reactivity. The outermost shell, containing the valence electrons, plays the most significant role in chemical bonding.
Carbon's Electronic Configuration and Valence Electrons
Carbon's atomic number is 6, meaning it possesses 6 protons and 6 electrons in its neutral state. These electrons are arranged in shells according to the Aufbau principle, which dictates that electrons fill the lowest energy levels first. Thus, carbon's electron configuration is 1s²2s²2p².
Let's break this down:
- 1s²: Two electrons occupy the 1s subshell, the lowest energy level.
- 2s²: Two electrons occupy the 2s subshell.
- 2p²: Two electrons occupy the 2p subshell.
The outermost shell of carbon is the second shell (L shell). This shell contains a total of four electrons (two in the 2s subshell and two in the 2p subshell). Therefore, carbon has four valence electrons.
The Significance of Carbon's Four Valence Electrons
The presence of four valence electrons is the key to carbon's exceptional versatility in forming chemical bonds. This allows carbon to:
-
Form four covalent bonds: Carbon atoms readily share their four valence electrons with other atoms to achieve a stable octet (eight electrons) in their outermost shell, satisfying the octet rule. This capacity for forming four strong covalent bonds is central to the vast diversity of organic compounds.
-
Form single, double, and triple bonds: Carbon can form single bonds (sharing one electron pair), double bonds (sharing two electron pairs), and triple bonds (sharing three electron pairs) with other atoms, leading to variations in molecular structure and properties. This ability to form multiple bonds contributes to the complexity and richness of organic molecules.
-
Catination: Carbon's ability to bond strongly with other carbon atoms (catination) is unique and crucial for the formation of long chains, branched structures, and rings, all fundamental structural motifs in organic chemistry. This capacity allows for the creation of incredibly complex and large molecules.
-
Isomerism: The versatile bonding of carbon leads to the possibility of isomerism, where molecules with the same chemical formula exhibit different structures and properties. This adds another layer of complexity to organic chemistry and contributes to the enormous variety of organic compounds.
Carbon's Role in Organic Chemistry and Biology
Carbon's four valence electrons are the foundation of organic chemistry, the branch of chemistry dedicated to the study of carbon-containing compounds. The vast array of organic molecules, from simple hydrocarbons to complex biomolecules, owes its existence to carbon's unique bonding capabilities.
Here are some key examples highlighting the importance of carbon's valence electrons in various contexts:
-
Hydrocarbons: These are the simplest organic molecules, consisting solely of carbon and hydrogen atoms. The carbon atoms form the backbone of the molecule, with hydrogen atoms bonded to satisfy carbon's four valence electrons. Examples include methane (CH₄), ethane (C₂H₆), and propane (C₃H₈).
-
Carbohydrates: These essential biomolecules are composed of carbon, hydrogen, and oxygen atoms. The carbon atoms form the backbone of carbohydrate structures, with hydroxyl groups (-OH) and other functional groups attached. Sugars, starches, and cellulose are examples of carbohydrates.
-
Proteins: Proteins are complex biomolecules composed of amino acids, which contain carbon, hydrogen, oxygen, nitrogen, and sometimes sulfur. The carbon atoms form the backbone of the amino acid structure, with amino and carboxyl groups attached. Proteins are essential for various biological functions.
-
Lipids: Lipids, including fats and oils, are composed of carbon, hydrogen, and oxygen atoms. They contain long hydrocarbon chains, where carbon atoms form the backbone and are bonded to hydrogen atoms. Lipids serve as energy storage molecules and structural components of cells.
-
Nucleic acids: DNA and RNA, the genetic materials of life, are composed of nucleotides. Nucleotides contain carbon, hydrogen, oxygen, nitrogen, and phosphorus atoms. The carbon atoms form the backbone of the nucleotide structure, with nitrogenous bases and phosphate groups attached.
In each of these examples, the presence of four valence electrons in carbon atoms is essential for building the diverse structures of organic molecules, making carbon the central atom of life itself.
The Periodic Table and Predicting Valence Electrons
The periodic table provides a powerful tool for predicting the number of valence electrons an element possesses. The group number (vertical column) in the periodic table often corresponds to the number of valence electrons for main group elements (Groups 1-18). Carbon belongs to Group 14, also known as Group IVA. Elements in Group 14 generally have four valence electrons. Therefore, by simply knowing carbon's position in the periodic table, we can readily deduce that it has four valence electrons.
Exceptions and Advanced Concepts
While the octet rule and the simple model of electron shells provide a good understanding of carbon's bonding, there are some exceptions and more nuanced concepts to consider:
-
Expanded octets: In some cases, elements in the third period and beyond can accommodate more than eight electrons in their valence shell. This phenomenon, known as expanded octets, can occur in certain compounds containing carbon, but it's less common.
-
Hypervalency: Hypervalent molecules have central atoms that appear to exceed the typical maximum number of covalent bonds. While carbon rarely exhibits hypervalency, understanding the concept expands our appreciation of bonding complexities beyond the simple octet rule.
-
Molecular Orbital Theory: A more sophisticated approach to understanding chemical bonding involves molecular orbital theory (MOT). MOT provides a more accurate description of electron distribution in molecules, accounting for the interaction of atomic orbitals to form molecular orbitals. This theory offers deeper insights into bonding, but for a basic understanding of carbon's valence electrons, the simpler shell model suffices.
Conclusion: The Importance of Carbon's Four Valence Electrons
The number of electrons in the outer shell of an atom determines its chemical behavior and bonding capacity. Carbon, with its four valence electrons, stands out as a uniquely versatile element. This characteristic allows carbon to form strong covalent bonds with a wide range of atoms, including itself, leading to the incredible diversity of organic compounds essential for life and numerous applications. The understanding of carbon's electron configuration is crucial not only for organic chemists but also for anyone interested in the fundamental building blocks of our world. The simplicity of the electron configuration belies the immense complexity and richness that arises from its four valence electrons, making carbon truly the exceptional element it is. Its central role in organic chemistry and biology highlights the profound significance of those four electrons in shaping the natural world and the materials we use every day.
Latest Posts
Latest Posts
-
How Many Feet In 70 Inches
Apr 01, 2025
-
What Is Standard Form Of A Polynomial
Apr 01, 2025
-
El 5 Por Ciento De 1000
Apr 01, 2025
-
How Many Feet Is 110 In
Apr 01, 2025
-
36 7 C To F In Fahrenheit
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Are In The Outer Shell Of Carbon . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
