How Many Electrons Do Chlorine Have
Kalali
Apr 01, 2025 · 5 min read
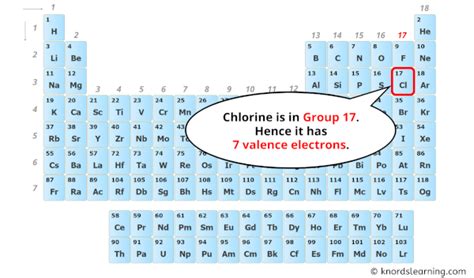
Table of Contents
How Many Electrons Does Chlorine Have? A Deep Dive into Atomic Structure
Chlorine, a vibrant yellow-green gas, plays a crucial role in various aspects of our lives, from purifying water to producing essential chemicals. Understanding its atomic structure, particularly the number of electrons it possesses, is key to grasping its chemical behavior and reactivity. This comprehensive article delves into the fascinating world of chlorine's electron configuration, exploring its implications in bonding, oxidation states, and its overall significance in chemistry.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before we delve into the specifics of chlorine, let's establish a fundamental understanding of atomic structure. An atom is the basic unit of a chemical element. It consists of three subatomic particles:
- Protons: Positively charged particles located in the atom's nucleus. The number of protons defines the atomic number of an element and determines its identity.
- Neutrons: Neutrally charged particles also residing in the nucleus. They contribute to the atom's mass but not its charge.
- Electrons: Negatively charged particles that orbit the nucleus in energy levels or shells. The number of electrons generally equals the number of protons in a neutral atom, ensuring an overall neutral charge.
The arrangement of electrons in these shells dictates an atom's chemical properties and how it interacts with other atoms.
Chlorine's Position on the Periodic Table: Unveiling its Electron Configuration
Chlorine (Cl) is located in Group 17 (also known as Group VIIA or the halogens) and Period 3 of the periodic table. Its atomic number is 17, meaning a neutral chlorine atom contains 17 protons. Consequently, a neutral chlorine atom also possesses 17 electrons.
Electron Shell Configuration: The Key to Chlorine's Reactivity
These 17 electrons are distributed across different energy levels or shells. The electron configuration of chlorine is written as 1s²2s²2p⁶3s²3p⁵. Let's break this down:
- 1s²: Two electrons occupy the first energy level (shell), specifically the 's' subshell.
- 2s²: Two electrons fill the 's' subshell of the second energy level.
- 2p⁶: Six electrons fill the 'p' subshell of the second energy level. The 'p' subshell can hold a maximum of six electrons.
- 3s²: Two electrons occupy the 's' subshell of the third energy level.
- 3p⁵: Five electrons occupy the 'p' subshell of the third energy level. This subshell is not fully filled, with one electron short of its maximum capacity of six.
This incomplete outermost shell (the valence shell) is the reason behind chlorine's high reactivity. Atoms strive for stability, which is typically achieved by having a full valence shell (usually eight electrons, following the octet rule). Chlorine readily gains one electron to achieve this stable configuration, forming a chloride ion (Cl⁻) with a full 3p subshell and a total of 18 electrons.
Chlorine's Reactivity and Chemical Bonding: The Role of Electrons
Chlorine's strong tendency to gain an electron makes it highly reactive. It readily forms ionic bonds with metals and covalent bonds with nonmetals.
Ionic Bonding: An Electron's Transfer
In ionic bonding, chlorine readily accepts an electron from a metal atom, such as sodium (Na). Sodium, with one electron in its outermost shell, readily loses this electron to achieve a stable configuration. This transfer of an electron creates oppositely charged ions: the positively charged sodium ion (Na⁺) and the negatively charged chloride ion (Cl⁻). The electrostatic attraction between these ions forms the ionic bond, resulting in the formation of sodium chloride (NaCl), commonly known as table salt.
Covalent Bonding: Electron Sharing
With nonmetals, chlorine forms covalent bonds by sharing electrons. For example, in chlorine gas (Cl₂), two chlorine atoms share one pair of electrons, completing each other's valence shells. Each chlorine atom effectively "sees" eight electrons in its valence shell through this sharing, achieving stability.
Chlorine's Oxidation States: A Reflection of Electron Transfer
The concept of oxidation states reflects the apparent charge of an atom in a compound based on electron transfer. Chlorine typically exhibits a negative oxidation state of -1 when it gains an electron to form the chloride ion (Cl⁻), as seen in NaCl. However, chlorine can also exhibit positive oxidation states in compounds with more electronegative elements like oxygen, where it partially loses electrons. For example, in chlorine dioxide (ClO₂), chlorine has an oxidation state of +4.
Chlorine's Importance: Applications in Diverse Fields
Chlorine's unique properties, stemming from its electron configuration and reactivity, make it essential in various applications:
- Water Purification: Chlorine is widely used as a disinfectant in water treatment plants to kill harmful bacteria and viruses, ensuring safe drinking water.
- Chemical Industry: Chlorine is a crucial building block in the production of numerous chemicals, including PVC (polyvinyl chloride) plastics, solvents, and refrigerants.
- Medicine: Chlorine compounds are used in disinfectants and some medications.
- Bleaching: Chlorine-based bleaching agents are used to whiten fabrics and paper.
Isotopes of Chlorine: Variations in Neutron Count, Not Electron Count
While the number of electrons in a neutral chlorine atom is always 17, chlorine exists in two stable isotopes: chlorine-35 (³⁵Cl) and chlorine-37 (³⁷Cl). Isotopes are atoms of the same element with the same number of protons but differing numbers of neutrons. This difference in neutron number affects the atom's mass but not its chemical properties, which are primarily determined by electron configuration. Both isotopes have 17 protons and 17 electrons in their neutral state.
Conclusion: The Significance of 17 Electrons
The seemingly simple number 17 – the number of electrons in a chlorine atom – holds the key to understanding its remarkable chemical behavior and wide-ranging applications. Its incomplete valence shell drives its high reactivity, leading to the formation of ionic and covalent bonds and a variety of important compounds. This understanding of chlorine's atomic structure highlights the fundamental principles of chemistry and the crucial role that electron configuration plays in determining an element's properties and behavior. Further exploration into the intricacies of electron shells and orbitals provides a deeper appreciation for the complexity and elegance of the atomic world. From purifying our water to creating essential materials, chlorine's 17 electrons profoundly impact our daily lives.
Latest Posts
Latest Posts
-
Describe The Cross Section Of The Rectangular Prism
Apr 02, 2025
-
7 Liters Is How Many Gallons
Apr 02, 2025
-
Is Baking Soda A Compound Element Or Mixture
Apr 02, 2025
-
What Is 177 Minutes In Hours And Minutes
Apr 02, 2025
-
Cuanto Es El 20 Por Ciento De 20
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Do Chlorine Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
