How Many Formula Units Are In 35.0 G Kno3
Kalali
Mar 27, 2025 · 4 min read
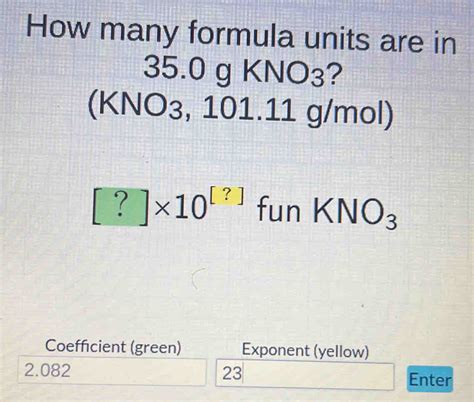
Table of Contents
How Many Formula Units Are in 35.0 g KNO₃? A Comprehensive Guide
This article will delve into the process of calculating the number of formula units in 35.0 grams of potassium nitrate (KNO₃). We'll explore the fundamental concepts, step-by-step calculations, and related considerations to provide a comprehensive understanding of this stoichiometry problem.
Understanding Moles and Avogadro's Number
Before we begin the calculation, let's establish the foundational concepts crucial to understanding stoichiometry:
Moles:
The mole (mol) is the cornerstone of chemical calculations. It represents a specific number of particles, be it atoms, molecules, ions, or formula units. One mole of any substance contains Avogadro's number of particles.
Avogadro's Number:
Avogadro's number is approximately 6.022 x 10²³. This immense number represents the number of particles in one mole of a substance. It's a constant, crucial for converting between macroscopic measurements (like grams) and the microscopic world of atoms and molecules.
Molar Mass:
The molar mass is the mass of one mole of a substance. It's expressed in grams per mole (g/mol). For KNO₃, we need to add the atomic masses of each element:
- Potassium (K): 39.10 g/mol
- Nitrogen (N): 14.01 g/mol
- Oxygen (O): 16.00 g/mol (x3 since there are three oxygen atoms)
Therefore, the molar mass of KNO₃ is: 39.10 + 14.01 + (3 * 16.00) = 101.11 g/mol
Calculating Formula Units in 35.0 g KNO₃
Now, let's tackle the problem of determining the number of formula units in 35.0 g of KNO₃. We'll approach this in a systematic manner, breaking it down into manageable steps:
Step 1: Convert Grams to Moles
First, we need to convert the given mass (35.0 g) of KNO₃ into moles using its molar mass (101.11 g/mol):
Moles of KNO₃ = (Mass of KNO₃) / (Molar mass of KNO₃)
Moles of KNO₃ = (35.0 g) / (101.11 g/mol) ≈ 0.346 moles
Step 2: Convert Moles to Formula Units
Now that we have the number of moles, we can use Avogadro's number to convert moles of KNO₃ into the number of formula units:
Formula units of KNO₃ = (Moles of KNO₃) x (Avogadro's number)
Formula units of KNO₃ = (0.346 mol) x (6.022 x 10²³ formula units/mol) ≈ 2.08 x 10²³ formula units
Therefore, there are approximately 2.08 x 10²³ formula units in 35.0 g of KNO₃.
Expanding on the Concepts: Further Exploration
Let's explore some related concepts and scenarios to solidify our understanding:
Significance of Formula Units:
The term "formula unit" is used for ionic compounds like KNO₃. Unlike molecular compounds which exist as discrete molecules, ionic compounds exist as a lattice of ions. A formula unit represents the simplest whole-number ratio of ions in the ionic compound.
Dealing with Different Units:
The process remains the same even if the mass is given in different units (e.g., milligrams, kilograms). The key is to convert the mass to grams before calculating the number of moles.
Calculating for Other Compounds:
The method described above is applicable to calculating formula units or molecules for any compound. You simply need to determine the molar mass of the specific compound you're working with.
Practical Applications:
Understanding these calculations is vital in various fields:
- Chemistry: Stoichiometric calculations are fundamental in chemical reactions, determining reactant quantities, and predicting product yields.
- Pharmacology: Accurate dosage calculations in drug preparation and administration rely on precise molar mass and Avogadro's number applications.
- Material Science: Designing materials with specific properties requires understanding the relationships between mass, moles, and the number of constituent particles.
- Environmental Science: Monitoring pollutant concentrations and assessing environmental impact involve these fundamental calculations.
Potential Sources of Error and Precision
While our calculations are straightforward, it's important to acknowledge potential sources of error:
- Significant Figures: Maintaining the correct number of significant figures throughout the calculation is crucial for accuracy. In our example, the final answer should reflect the precision of the given mass (35.0 g).
- Molar Mass Precision: The atomic masses used to calculate the molar mass of KNO₃ are themselves approximations. More precise atomic masses can lead to slightly different results.
- Avogadro's Number Approximation: Avogadro's number is an approximation. Using a more precise value can affect the final answer, though the difference is usually negligible for most applications.
Conclusion: Mastering Stoichiometry
This comprehensive guide demonstrates the step-by-step process of determining the number of formula units in a given mass of potassium nitrate. By understanding the concepts of moles, molar mass, and Avogadro's number, we can confidently tackle stoichiometry problems and apply these principles in various scientific and practical contexts. Remember to pay close attention to significant figures and understand the inherent approximations in the constants used in the calculations. This understanding is crucial for accurate results and successful applications of stoichiometry in various fields. The ability to perform these calculations is a vital skill for anyone pursuing studies or careers in science and related fields. Further practice with diverse compounds and scenarios will solidify this understanding and enable you to confidently tackle more complex stoichiometry problems.
Latest Posts
Latest Posts
-
Is Ductility A Physical Or Chemical Property
Mar 30, 2025
-
Lowest Common Factor Of 3 And 4
Mar 30, 2025
-
How Much Is 1 4 Cup In Ounces
Mar 30, 2025
-
What Percentage Is 1 In 6
Mar 30, 2025
-
What Is The Thickness Of The Outer Core
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about How Many Formula Units Are In 35.0 G Kno3 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
