How Many Molecules In 2.0 Moles
Kalali
Mar 27, 2025 · 5 min read
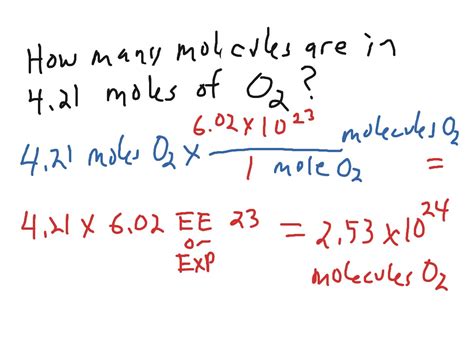
Table of Contents
How Many Molecules in 2.0 Moles? A Deep Dive into Avogadro's Number
Understanding the relationship between moles, molecules, and Avogadro's number is fundamental to chemistry. This article will delve deep into this concept, explaining not just the answer to the title question but also providing a comprehensive understanding of the underlying principles and their broader applications. We'll explore Avogadro's number itself, its significance, and how it allows us to bridge the macroscopic world of grams and moles with the microscopic world of atoms and molecules.
Understanding Moles: The Chemist's Dozen
Before we tackle the central question, let's clarify what a mole represents. A mole (mol) is a fundamental unit in chemistry, representing a specific number of entities – be it atoms, molecules, ions, or even photons. This number, known as Avogadro's number, is approximately 6.022 x 10²³. Think of it like a chemist's "dozen," but instead of 12, we have a vastly larger quantity.
One mole of any substance contains Avogadro's number of its constituent particles. This provides a convenient way to relate the mass of a substance (which we can measure) to the number of particles it contains (which we can't directly count).
Avogadro's Number: The Bridge Between the Macro and Micro
Avogadro's number (N<sub>A</sub>) is a constant that plays a crucial role in chemistry. It's named after Amedeo Avogadro, an Italian scientist who proposed that equal volumes of gases at the same temperature and pressure contain the same number of molecules (Avogadro's Law). This groundbreaking idea was instrumental in developing our understanding of atomic weights and molar masses.
The value of Avogadro's number is not arbitrary; it's carefully defined based on the number of atoms in 12 grams of carbon-12. This ensures a consistent and reliable standard for measurements involving moles.
Calculating Molecules in 2.0 Moles
Now, let's get to the core of the question: how many molecules are present in 2.0 moles of a substance? The answer is straightforward:
- One mole contains 6.022 x 10²³ molecules.
- Therefore, 2.0 moles contain 2.0 x (6.022 x 10²³ molecules) = 1.2044 x 10²⁴ molecules.
This calculation assumes we are dealing with a substance composed of molecules. For substances composed of individual atoms (like noble gases), the calculation would yield the number of atoms instead of molecules.
Beyond the Simple Calculation: Factors to Consider
While the calculation above is simple, it’s crucial to understand the assumptions and potential complexities:
1. The Nature of the Substance:
The calculation directly applies to substances made up of discrete molecules. For ionic compounds (like NaCl), the calculation would give the number of formula units (NaCl units in this case) rather than molecules, as ionic compounds don't exist as individual molecules in the same way covalent compounds do. For elements existing as individual atoms (like Helium or Argon), the calculation will give the number of atoms present.
2. Purity of the Substance:
The calculation assumes the substance is 100% pure. Impurities will decrease the actual number of molecules present for a given number of moles. The purity of a substance is crucial for accurate calculations in real-world scenarios.
3. Ideal vs. Real Gases:
For gases, the calculation assumes ideal gas behavior. At high pressures or low temperatures, gases deviate from ideal behavior due to intermolecular forces and molecular volume, leading to slight discrepancies in the actual number of molecules.
4. Significance of Avogadro's Number in Calculations:
Avogadro's number is pivotal in various chemical calculations:
- Determining the number of particles: Given the number of moles, we can quickly calculate the number of atoms or molecules present.
- Mass-mole conversions: By combining Avogadro's number with molar mass (the mass of one mole of a substance), we can easily convert between mass and the number of moles.
- Stoichiometric Calculations: Avogadro's number allows us to understand and perform calculations based on the mole ratios in balanced chemical equations, enabling predictions of reactant and product quantities.
- Concentration calculations: Avogadro's number is key in calculating concentrations in solutions, often expressed as molarity (moles per liter).
Practical Applications of Mole Calculations
The concept of moles and Avogadro's number extends far beyond theoretical calculations. Its applications are crucial in various fields:
- Pharmaceutical Industry: Accurate mole calculations are essential for formulating drugs, ensuring precise dosages and consistent drug efficacy.
- Materials Science: Determining the precise stoichiometry of materials is critical for controlling their properties, and mole calculations are vital in this process.
- Environmental Science: Analyzing the concentration of pollutants in the environment often involves mole calculations.
- Food Science: Understanding the composition of food products often requires precise mole calculations to ensure nutritional content and food safety.
- Analytical Chemistry: Many analytical techniques rely on the precise measurement and calculation of the number of moles of a substance present in a sample.
Expanding the Concept: Molar Mass and Molar Volume
Two closely related concepts to moles are molar mass and molar volume:
- Molar Mass: The molar mass of a substance is the mass of one mole of that substance. It's expressed in grams per mole (g/mol) and can be calculated by summing the atomic masses of all atoms in the chemical formula of the compound.
- Molar Volume: The molar volume of a substance, particularly for gases, is the volume occupied by one mole of that substance under standard conditions (usually 0°C and 1 atm pressure). At standard temperature and pressure (STP), the molar volume of an ideal gas is approximately 22.4 liters.
Conclusion: The Power of Avogadro's Number
Avogadro's number and the concept of moles are fundamental pillars of chemistry, providing a bridge between the macroscopic world of measurable quantities and the microscopic world of atoms and molecules. Understanding how to calculate the number of molecules in a given number of moles is not just a matter of academic exercise but a crucial skill applicable across diverse scientific and industrial fields. The ability to perform these calculations accurately ensures precision and accuracy in various applications, from drug development to environmental monitoring. While the calculation for 2.0 moles might seem simple – 1.2044 x 10²⁴ molecules – the underlying principles and the broader implications of this concept are vast and incredibly impactful. This deep dive highlights the significance of Avogadro's number and its pervasive role in shaping our understanding and manipulation of the matter around us.
Latest Posts
Latest Posts
-
What Does Mu Mean In Statistics
Mar 30, 2025
-
What Type Of Organism Is The Grass
Mar 30, 2025
-
How Much Is 2 3 Cup In Ounces
Mar 30, 2025
-
Which Method Helps Prevent Communicable Diseases
Mar 30, 2025
-
What Is A 39 Out Of 50
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about How Many Molecules In 2.0 Moles . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
