How Many Valence Electrons Are In Ca
Kalali
Mar 15, 2025 · 5 min read
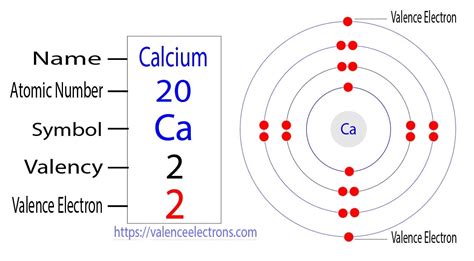
Table of Contents
How Many Valence Electrons Are in Ca? Understanding Calcium's Reactivity
Calcium (Ca), a vital element for human health and a cornerstone in various industrial applications, holds a fascinating position in the periodic table. Understanding its chemical behavior hinges on knowing its valence electrons – the outermost electrons responsible for its bonding capabilities and reactivity. This article delves deep into the electronic structure of calcium, explaining how many valence electrons it possesses and how this number dictates its chemical properties. We’ll explore its position in the periodic table, its electron configuration, and its role in forming chemical compounds.
Understanding Valence Electrons
Before we pinpoint the number of valence electrons in calcium, let's clarify what valence electrons are. Valence electrons are the electrons located in the outermost shell (or energy level) of an atom. They are the electrons most readily involved in chemical bonding, determining an element's reactivity and the types of bonds it can form. The number of valence electrons directly influences an element's chemical properties, such as its tendency to gain, lose, or share electrons to achieve a stable electron configuration.
Calcium's Position in the Periodic Table
Calcium resides in Group 2 (also known as the alkaline earth metals) and Period 4 of the periodic table. This location provides crucial information about its electronic structure. The group number (2) indicates that calcium has two valence electrons. The period number (4) tells us that its outermost electrons are located in the fourth energy level.
Calcium's Electron Configuration
The electron configuration of an atom describes the arrangement of electrons in its energy levels and subshells. For calcium (atomic number 20), the electron configuration is 1s²2s²2p⁶3s²3p⁶4s².
Let's break this down:
- 1s²: Two electrons in the first energy level, first subshell (s).
- 2s²: Two electrons in the second energy level, first subshell (s).
- 2p⁶: Six electrons in the second energy level, second subshell (p).
- 3s²: Two electrons in the third energy level, first subshell (s).
- 3p⁶: Six electrons in the third energy level, second subshell (p).
- 4s²: Two electrons in the fourth energy level, first subshell (s).
The outermost shell is the fourth energy level, containing the two 4s electrons. Therefore, calcium has two valence electrons.
Why Two Valence Electrons are Significant for Calcium's Reactivity
Having two valence electrons makes calcium highly reactive, particularly with nonmetals. Calcium readily loses these two electrons to achieve a stable octet (eight electrons) in its outermost shell, mimicking the electron configuration of the noble gas argon. This process of losing electrons is known as oxidation, and it results in the formation of a +2 ion (Ca²⁺).
Chemical Reactions Involving Calcium
Calcium's reactivity is evident in its various chemical reactions. Here are some examples:
Reaction with Water:
Calcium reacts vigorously with water, producing calcium hydroxide (Ca(OH)₂) and hydrogen gas (H₂). The reaction is exothermic, releasing heat. This reaction demonstrates calcium's tendency to lose its two valence electrons to form the Ca²⁺ ion.
Ca(s) + 2H₂O(l) → Ca(OH)₂(aq) + H₂(g)
Reaction with Oxygen:
Calcium readily reacts with oxygen in the air to form calcium oxide (CaO), commonly known as quicklime. This reaction is also exothermic.
2Ca(s) + O₂(g) → 2CaO(s)
Reaction with Acids:
Calcium reacts with acids such as hydrochloric acid (HCl) to produce calcium chloride (CaCl₂), hydrogen gas, and heat.
Ca(s) + 2HCl(aq) → CaCl₂(aq) + H₂(g)
Formation of Ionic Compounds:
Due to its tendency to lose electrons, calcium primarily forms ionic compounds. These compounds are formed through electrostatic attraction between the positively charged calcium ion (Ca²⁺) and negatively charged ions of other elements. For example, calcium chloride (CaCl₂) is an ionic compound formed by the electrostatic attraction between Ca²⁺ and Cl⁻ ions.
Calcium's Importance in Biological Systems
Calcium plays a crucial role in various biological processes. It is essential for:
- Bone and teeth formation: Calcium is the primary mineral component of bones and teeth, providing structural strength and support.
- Muscle contraction: Calcium ions are involved in muscle contraction and relaxation.
- Nerve impulse transmission: Calcium ions play a key role in transmitting nerve impulses.
- Blood clotting: Calcium is essential for blood clotting.
- Enzyme activation: Many enzymes require calcium ions for their activity.
Calcium's Industrial Applications
Besides its biological importance, calcium finds widespread use in various industries:
- Construction: Calcium compounds like limestone (CaCO₃) and gypsum (CaSO₄·2H₂O) are widely used in construction materials such as cement, plaster, and mortar.
- Metallurgy: Calcium is used as a reducing agent in the extraction of certain metals from their ores.
- Agriculture: Calcium is an essential nutrient for plants and is often added to fertilizers.
- Food industry: Calcium compounds are used as food additives, such as calcium carbonate in baking powder.
Conclusion: The Significance of Calcium's Two Valence Electrons
The presence of two valence electrons in calcium's outermost shell is the key to understanding its reactivity and its diverse applications. This characteristic leads to its tendency to lose these electrons, forming the stable Ca²⁺ ion and driving its participation in various chemical reactions and biological processes. From building strong bones and teeth to being a crucial component in cement, calcium's chemical properties, all stemming from its two valence electrons, make it an indispensable element in both biological and industrial contexts. Its readily available ions, a direct consequence of its electron configuration, underpins its critical roles in maintaining life and driving technological advancements. Understanding valence electrons, therefore, is fundamental to grasping the chemical behavior and significance of elements like calcium.
Latest Posts
Latest Posts
-
What Is 24 40 As A Percentage
Mar 15, 2025
-
What Are The Two Parts Of Solution
Mar 15, 2025
-
What Occurs When A Gas Is Compressed
Mar 15, 2025
-
How Do You Convert Moles To Liters
Mar 15, 2025
-
When The Speed Of An Object Is Doubled Its Momentum
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Are In Ca . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
