What Occurs When A Gas Is Compressed
Kalali
Mar 15, 2025 · 6 min read
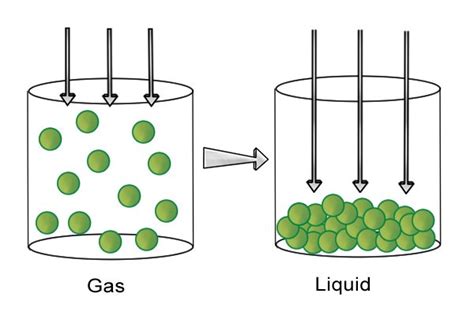
Table of Contents
What Happens When a Gas is Compressed? A Deep Dive into Gas Compression
Understanding what happens when a gas is compressed is crucial across numerous scientific and engineering disciplines. From refrigeration systems and internal combustion engines to industrial processes and even the physics of stars, gas compression plays a vital role. This article will explore the thermodynamic principles governing gas compression, detailing the changes in pressure, temperature, volume, and energy, and examining various methods and applications of this fundamental process.
The Fundamental Principles: Pressure, Volume, and Temperature
The behavior of gases during compression is primarily governed by the laws of thermodynamics, particularly the ideal gas law and its variations. The ideal gas law, expressed as PV = nRT, provides a foundational relationship between pressure (P), volume (V), number of moles (n), ideal gas constant (R), and absolute temperature (T). This equation assumes that gas molecules have negligible size and intermolecular forces, an assumption that holds reasonably well for many gases under moderate conditions.
Isothermal Compression: Constant Temperature
In isothermal compression, the temperature of the gas remains constant throughout the process. This is typically achieved by allowing heat to transfer freely between the gas and its surroundings. As the volume decreases, the pressure increases proportionally, according to Boyle's Law (P₁V₁ = P₂V₂ at constant temperature and moles). No net change in internal energy occurs, as the work done on the gas is balanced by the heat transferred to the surroundings. The process is illustrated by a curve on a P-V diagram (pressure-volume diagram).
Adiabatic Compression: No Heat Exchange
In contrast to isothermal compression, adiabatic compression involves no heat exchange between the gas and its surroundings. This means the system is perfectly insulated. As the gas is compressed, both its pressure and temperature increase. The work done on the gas increases its internal energy, manifested as a rise in temperature. The relationship between pressure and volume in an adiabatic process follows the equation: P₁V₁<sup>γ</sup> = P₂V₂<sup>γ</sup>, where γ (gamma) represents the ratio of specific heats (Cp/Cv). This ratio depends on the nature of the gas. The adiabatic process is represented by a steeper curve on a P-V diagram compared to an isothermal process.
Isobaric Compression: Constant Pressure
Isobaric compression occurs when the pressure of the gas remains constant during compression. This usually requires a controlled system where the external pressure is maintained at a constant value. As the volume decreases, the temperature will proportionally decrease. To maintain constant pressure, heat must be removed from the system. On a P-V diagram, this process is represented by a horizontal line.
The Role of Energy: Work and Heat
Compression is fundamentally a process of doing work on the gas. The work done (W) during compression is given by the integral of pressure with respect to volume: W = ∫PdV. This integral's value depends on the path taken on the P-V diagram. For example, the work done in an isothermal process differs from that in an adiabatic process due to different pressure-volume relationships.
The first law of thermodynamics, ΔU = Q - W, states that the change in internal energy (ΔU) of a gas is equal to the heat added (Q) minus the work done by the gas (W). During compression, work is done on the gas (W is negative), leading to an increase in internal energy and, consequently, an increase in temperature (except for isothermal compression).
Factors Affecting Gas Compression: Gas Properties and Equipment
The behavior of a gas during compression isn't solely determined by the type of compression (isothermal, adiabatic, isobaric). Several other factors play significant roles:
1. Gas Properties:
- Molecular weight: Heavier gases require more energy to compress than lighter gases.
- Specific heat ratio (γ): This impacts the temperature change during adiabatic compression.
- Compressibility factor (Z): This accounts for deviations from ideal gas behavior, particularly at high pressures and low temperatures. Real gases exhibit intermolecular forces and molecular volumes, unlike ideal gases.
- Initial temperature and pressure: These set the starting point for the compression process.
2. Compression Equipment:
Different types of compressors are used for various applications, each with its own characteristics affecting the compression process:
- Reciprocating compressors: These use pistons to compress the gas in a cyclical manner. They're suitable for high-pressure applications but can be less efficient than other types.
- Rotary compressors: These use rotating elements (like screws or lobes) to compress the gas. They are generally more efficient at lower pressures than reciprocating compressors.
- Centrifugal compressors: These use rotating impellers to accelerate the gas, converting kinetic energy to pressure energy. They are well-suited for high-volume, low-pressure applications.
Applications of Gas Compression
Gas compression is a ubiquitous process with a wide array of applications:
- Refrigeration: Refrigerant gases are compressed to increase their temperature and pressure, facilitating heat transfer to the surroundings.
- Internal combustion engines: The compression stroke in an engine raises the temperature and pressure of the air-fuel mixture, initiating combustion.
- Industrial processes: Many industrial processes, such as the synthesis of ammonia and the liquefaction of natural gas, require gas compression.
- Natural gas pipelines: Natural gas is compressed to increase its density and facilitate transportation through pipelines.
- Aerospace: Gas compression is essential in various aerospace applications, including rocket propulsion and pressurization systems.
- Pneumatic systems: Compressed air is widely used to power tools and machinery.
Challenges and Considerations
Gas compression isn't without its challenges:
- Energy consumption: Compressing gases requires significant energy input, especially for high-pressure applications. This has implications for both cost and environmental impact.
- Heat generation: Adiabatic compression generates heat, which can be detrimental if not managed properly. Cooling systems are often necessary to maintain acceptable temperatures.
- Equipment wear and tear: Compressors are subjected to considerable stress, leading to potential wear and tear. Regular maintenance is crucial.
- Safety concerns: High-pressure gases pose safety risks if not handled carefully. Strict safety protocols and equipment are essential.
Conclusion
Gas compression is a fundamental process with far-reaching implications across various scientific and engineering disciplines. Understanding the thermodynamic principles underlying compression, the factors influencing its efficiency, and the various methods and applications is crucial for optimizing processes and ensuring safe operation. As technology continues to advance, we can expect to see further innovations in gas compression technology, leading to increased efficiency, reduced energy consumption, and wider applications in diverse fields. Further exploration into specific types of compressors, advanced thermodynamic cycles, and the development of sustainable compression methods will continue to shape our understanding and applications of this essential process.
Latest Posts
Latest Posts
-
Angle Properties Of A Circle Outside The Circle
Mar 15, 2025
-
Which Graph Represents The Rational Function
Mar 15, 2025
-
Which Applies To The Collision Theory
Mar 15, 2025
-
Which Would Allow Humans To Access Groundwater
Mar 15, 2025
-
What Are The Common Multiples Of 15 And 25
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about What Occurs When A Gas Is Compressed . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
