How Many Valence Electrons Are In Cesium
Kalali
Mar 19, 2025 · 5 min read
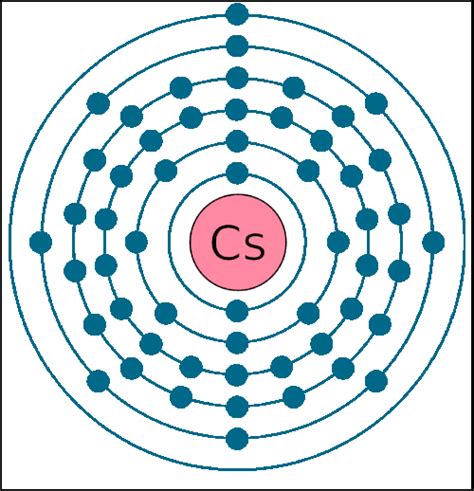
Table of Contents
How Many Valence Electrons Are in Cesium? A Deep Dive into Alkali Metals
Cesium, a fascinating element with a silvery-gold hue and remarkable properties, holds a unique position in the periodic table. Understanding its electronic structure, particularly the number of valence electrons, is crucial to comprehending its chemical behavior and its diverse applications. This article will comprehensively explore the valence electron count of cesium, delving into its atomic structure, periodic table placement, and the implications of its single valence electron.
Understanding Valence Electrons
Before we pinpoint the number of valence electrons in cesium, let's establish a firm understanding of what valence electrons actually are. Valence electrons are the electrons located in the outermost shell (or energy level) of an atom. These electrons are the primary players in chemical bonding, determining an element's reactivity and the types of compounds it can form. They are the most loosely held electrons, easily gained, lost, or shared during chemical reactions. The number of valence electrons largely dictates an element's chemical properties and its position within the periodic table.
Cesium's Position in the Periodic Table: A Key Indicator
Cesium (Cs) resides in Group 1 of the periodic table, also known as the alkali metals. This group is characterized by elements possessing a single valence electron in their outermost s-orbital. This consistent structural feature is directly responsible for the shared properties observed across alkali metals: high reactivity, low ionization energies, and the formation of +1 ions. The periodic table's organization provides a powerful tool for predicting the number of valence electrons for most elements, particularly those in the main group (s and p blocks).
Cesium's Electronic Configuration: Unveiling the Valence Electron
To definitively determine the number of valence electrons in cesium, we must examine its electronic configuration. The electronic configuration describes how electrons are distributed among the various energy levels and subshells within an atom. For cesium, with an atomic number of 55, the electronic configuration is:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s² 4d¹⁰ 5p⁶ 6s¹
Notice the outermost shell, the sixth energy level (n=6), contains only one electron in the 6s subshell. This solitary electron is the valence electron of cesium.
Deeper Dive into Electronic Configuration and Shells
Understanding electronic configuration requires familiarity with the principles governing electron filling. Electrons populate orbitals according to the Aufbau principle (filling lower energy levels first), Hund's rule (maximizing unpaired electrons within a subshell), and the Pauli exclusion principle (no two electrons can have the same quantum numbers). These rules govern the systematic filling of orbitals, resulting in the unique electronic configuration for each element. For cesium, the sequential filling of orbitals leads to that single electron residing in the outermost shell.
The Significance of Cesium's Single Valence Electron
The presence of just one valence electron profoundly impacts cesium's chemical behavior. This single electron is easily lost, resulting in the formation of a Cs⁺ ion, a cation with a +1 charge. This ease of electron loss explains cesium's high reactivity, particularly with nonmetals like halogens (fluorine, chlorine, bromine, iodine), where it readily forms ionic compounds. For example, cesium reacts vigorously with water, generating hydrogen gas and releasing a significant amount of heat.
Reactivity and Ionization Energy
Cesium's high reactivity is directly linked to its low ionization energy. Ionization energy is the energy required to remove an electron from an atom. Because the valence electron in cesium is relatively far from the nucleus and shielded by inner electrons, less energy is needed to remove it compared to elements with a higher number of valence electrons or smaller atomic radii. This low ionization energy contributes to cesium's readiness to participate in chemical reactions.
Applications Leveraging Cesium's Unique Properties
The unique properties stemming from its single valence electron make cesium valuable in various applications:
-
Atomic clocks: Cesium's precise atomic transitions are exploited in atomic clocks, providing incredibly accurate timekeeping. The cesium atom's specific frequency of microwave absorption is the basis for defining the second in the International System of Units (SI).
-
Oil and gas exploration: Cesium formate brines are utilized in enhanced oil recovery techniques, improving the extraction of hydrocarbons from underground reservoirs.
-
Medical imaging: Cesium-131, a radioactive isotope of cesium, has been used in certain medical imaging applications although its use has decreased due to safety and availability concerns.
-
Photoelectric cells: Cesium's ability to readily emit electrons when exposed to light makes it useful in photoelectric cells, converting light energy into electrical energy.
-
Scientific research: Cesium's unique properties and high reactivity make it invaluable for various research purposes in fields such as chemistry, physics, and materials science.
Comparing Cesium to Other Alkali Metals
Cesium shares many similarities with other alkali metals like lithium (Li), sodium (Na), potassium (K), rubidium (Rb), and francium (Fr). They all possess a single valence electron, resulting in similar chemical behaviors. However, cesium, being the largest of these elements, exhibits some unique characteristics. Its larger atomic radius and lower ionization energy make it the most reactive of the alkali metals, highlighting the influence of atomic structure on chemical properties.
Trends Across Alkali Metals
Moving down Group 1, the alkali metals exhibit increasing reactivity, lower ionization energy, and larger atomic radii. This trend is directly attributable to the increasing number of electron shells shielding the valence electron from the nucleus, making it easier to remove. Cesium, at the bottom of the group, exemplifies this trend to the highest degree.
Conclusion: Cesium's Single Valence Electron – A Defining Feature
In conclusion, cesium possesses one valence electron. This seemingly simple fact is fundamental to understanding its chemical behavior, reactivity, and wide range of applications. Its position in the periodic table, its electronic configuration, and its low ionization energy all converge to underscore the significant role of this single electron in shaping cesium's unique properties. Understanding valence electrons is key to comprehending the chemical world, and cesium provides a compelling example of how this fundamental concept impacts the properties and applications of an element. From atomic clocks to oil exploration, cesium's single valence electron drives its remarkable contributions to science and technology.
Latest Posts
Latest Posts
-
What Is 2 3 In A Fraction
Mar 19, 2025
-
How Many Ounces In 1 4 Liters
Mar 19, 2025
-
4 1 2 As A Decimal
Mar 19, 2025
-
How Do You Write 4 9 As A Decimal
Mar 19, 2025
-
How Will Cellular Respiration Affect Oxygen Levels
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Are In Cesium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
