How Many Valence Electrons Does Aluminium Have
Kalali
Mar 22, 2025 · 6 min read
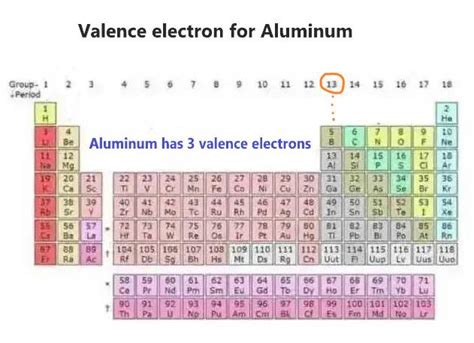
Table of Contents
How Many Valence Electrons Does Aluminium Have? A Deep Dive into Atomic Structure and Chemical Behavior
Aluminum, a ubiquitous metal found in everything from soda cans to aircraft parts, holds a fascinating place in the periodic table. Understanding its properties, particularly its number of valence electrons, is key to comprehending its reactivity and wide range of applications. This article will delve deep into the atomic structure of aluminum, explaining not just how many valence electrons it possesses but also why this number is so crucial to its chemical behavior. We'll explore its position within the periodic table, its electron configuration, and how its valence electrons dictate its bonding capabilities.
Understanding Valence Electrons: The Key to Chemical Reactions
Before we focus specifically on aluminum, let's establish a solid foundation. Valence electrons are the outermost electrons in an atom. These electrons are the ones most involved in chemical bonding, determining how an atom will interact with other atoms. They are the architects of chemical reactions, dictating whether an atom will readily gain, lose, or share electrons to achieve a stable electron configuration. This stable configuration, often referred to as a full octet (eight electrons in the outermost shell), is the driving force behind many chemical processes. Atoms strive to achieve this stability, and their valence electrons are the key players in this pursuit.
The Atomic Structure of Aluminum: Unpacking the Electron Configuration
Aluminum (Al) has an atomic number of 13, meaning it has 13 protons in its nucleus and, in a neutral atom, 13 electrons orbiting the nucleus. To understand its valence electrons, we need to examine its electron configuration. Electrons occupy specific energy levels or shells around the nucleus. These shells are designated by principal quantum numbers (n = 1, 2, 3, etc.), with each shell capable of holding a maximum number of electrons. The electron configuration of aluminum is 1s²2s²2p⁶3s²3p¹.
Let's break this down:
- 1s²: Two electrons occupy the first energy level (n=1) in the s orbital.
- 2s²: Two electrons occupy the second energy level (n=2) in the s orbital.
- 2p⁶: Six electrons occupy the second energy level (n=2) in the p orbital.
- 3s²: Two electrons occupy the third energy level (n=3) in the s orbital.
- 3p¹: One electron occupies the third energy level (n=3) in the p orbital.
Determining the Number of Valence Electrons in Aluminum
The valence electrons are those located in the outermost energy level. In aluminum's case, the outermost energy level is the third energy level (n=3). This level contains a total of three electrons: two in the 3s orbital and one in the 3p orbital.
Therefore, aluminum has three valence electrons.
The Significance of Aluminum's Three Valence Electrons
The presence of three valence electrons significantly influences aluminum's chemical behavior and properties. It explains why aluminum is:
-
Highly Reactive: Aluminum readily loses its three valence electrons to achieve a stable octet, forming a 3+ ion (Al³⁺). This ease of electron loss contributes to its high reactivity, particularly with oxidizing agents like oxygen. This explains why aluminum readily forms a protective oxide layer when exposed to air, preventing further oxidation.
-
Excellent Conductor of Electricity and Heat: The loosely held valence electrons are mobile and can easily move throughout the metallic lattice structure. This high electron mobility is responsible for aluminum's excellent conductivity, making it a crucial component in electrical wiring and heat exchangers.
-
Lightweight and Strong: The metallic bonding formed by the shared valence electrons results in a relatively strong and lightweight material, contributing to its wide use in various applications, including aerospace engineering.
-
Amphoteric Nature: Aluminum can react with both acids and bases, demonstrating amphoteric behavior. This is due to its ability to both lose electrons (acting as a metal) and accept electrons (acting as a non-metal under certain circumstances). This property makes it useful in various chemical processes.
Aluminum's Reactions and the Role of Valence Electrons
Let's examine some specific reactions involving aluminum to further illustrate the role of its valence electrons:
-
Reaction with Oxygen: Aluminum readily reacts with oxygen in the air, forming aluminum oxide (Al₂O₃). In this reaction, each aluminum atom loses three valence electrons to three oxygen atoms, forming strong ionic bonds. The resulting oxide layer is protective and prevents further oxidation.
-
Reaction with Acids: Aluminum reacts with acids like hydrochloric acid (HCl), producing aluminum chloride (AlCl₃) and hydrogen gas (H₂). The aluminum atoms lose their three valence electrons to the hydrogen ions in the acid, forming aluminum cations (Al³⁺) and liberating hydrogen gas.
-
Reaction with Bases: Aluminum also reacts with strong bases like sodium hydroxide (NaOH), forming aluminates and hydrogen gas. This reaction demonstrates aluminum's amphoteric nature.
Aluminum in the Periodic Table: Trends and Comparisons
Aluminum's position in the periodic table further clarifies its properties. It is located in Group 13 (or IIIA), also known as the boron group. Elements within this group generally have three valence electrons, exhibiting similar chemical behaviors. However, there are subtle differences due to variations in atomic size and other factors. Comparing aluminum to other Group 13 elements, like boron and gallium, reveals similarities in their tendency to form 3+ ions but also highlights differences in reactivity and other physical properties.
Applications of Aluminum and its Valence Electrons
The unique properties stemming from its three valence electrons make aluminum incredibly versatile. Its applications span numerous industries:
-
Packaging: Aluminum's resistance to corrosion and light weight make it ideal for food and beverage packaging (cans, foil).
-
Transportation: Used extensively in automobiles, aircraft, and trains due to its strength-to-weight ratio.
-
Construction: Aluminum alloys are employed in building structures, bridges, and other infrastructure projects.
-
Electrical Applications: Its excellent conductivity makes it essential in electrical wiring, transformers, and other electrical components.
-
Consumer Products: Found in a vast range of consumer products, from kitchen utensils to sporting goods.
-
Industrial Applications: Used in various industrial processes, including chemical manufacturing and heat exchangers.
Conclusion: Valence Electrons and the Versatility of Aluminum
The three valence electrons of aluminum are the key to understanding its remarkable properties and widespread applications. Its ability to readily lose these electrons dictates its reactivity, conductivity, and the formation of strong metallic bonds. This deep understanding of its atomic structure allows scientists and engineers to harness its unique characteristics for a wide array of innovative uses, making aluminum a truly indispensable element in modern society. The study of aluminum's valence electrons serves as a powerful example of how the fundamental principles of chemistry underpin the development and application of materials in our daily lives. From the humble soda can to the sophisticated components of an aircraft, aluminum’s success story is inextricably linked to the behavior of its three outermost electrons.
Latest Posts
Latest Posts
-
What Happens To The Volume Of A Gas During Compression
Mar 22, 2025
-
Oxidation State Of Sulfur In H2so4
Mar 22, 2025
-
Food Web Of Tropical Rainforest Biome
Mar 22, 2025
-
A Force Acting On An Object Does No Work If
Mar 22, 2025
-
Features Of A Type Iv Flotation Device
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Does Aluminium Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
