How Many Valence Electrons Does N Have
Kalali
Mar 25, 2025 · 6 min read
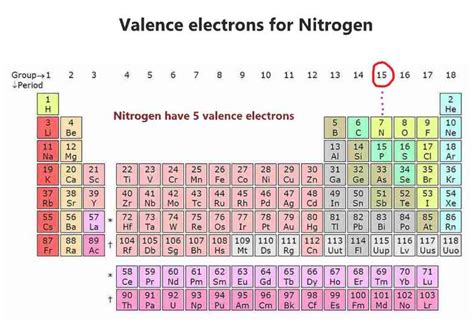
Table of Contents
How Many Valence Electrons Does Nitrogen (N) Have? A Deep Dive into Atomic Structure and Chemical Bonding
Nitrogen, a crucial element for life as we know it, plays a vital role in various biological and industrial processes. Understanding its atomic structure, particularly the number of valence electrons, is key to comprehending its chemical behavior and reactivity. This article will delve into the specifics of nitrogen's electronic configuration, explaining how to determine its valence electrons and highlighting the implications of this number in its chemical bonding and overall properties.
Understanding Valence Electrons: The Key to Reactivity
Before focusing on nitrogen, let's establish a foundational understanding of valence electrons. Valence electrons are the electrons located in the outermost shell (or energy level) of an atom. These electrons are the ones most involved in chemical bonding, determining an element's reactivity and the types of bonds it can form. The number of valence electrons significantly influences an element's properties, such as its electronegativity, ionization energy, and the formation of ionic or covalent bonds.
Atoms strive to achieve a stable electron configuration, often resembling that of a noble gas. This stability is typically achieved by having a full outermost shell, often containing eight electrons (the octet rule, though there are exceptions). Atoms with fewer than eight valence electrons tend to react readily to gain, lose, or share electrons to achieve this stable configuration.
Determining Nitrogen's Electronic Configuration
Nitrogen's atomic number is 7, meaning it has 7 protons and 7 electrons in a neutral atom. To determine the number of valence electrons, we need to understand its electronic configuration, which describes how these electrons are distributed among the different energy levels and subshells.
The electronic configuration of nitrogen is 1s²2s²2p³. Let's break this down:
- 1s²: This represents two electrons in the first energy level (n=1), specifically in the 's' subshell. The 's' subshell can hold a maximum of two electrons.
- 2s²: This indicates two electrons in the second energy level (n=2), in the 's' subshell.
- 2p³: This signifies three electrons in the second energy level (n=2), within the 'p' subshell. The 'p' subshell can accommodate a maximum of six electrons.
Nitrogen's Valence Electrons: The Answer
From the electronic configuration (1s²2s²2p³), we can clearly see that the outermost shell of nitrogen is the second energy level (n=2). This shell contains a total of five electrons (two from the 2s subshell and three from the 2p subshell). Therefore, nitrogen (N) has five valence electrons.
The Significance of Five Valence Electrons
The presence of five valence electrons profoundly impacts nitrogen's chemical behavior:
-
Covalent Bonding: Nitrogen readily forms covalent bonds, sharing its valence electrons with other atoms to achieve a stable octet. This is because gaining or losing three electrons to achieve a noble gas configuration would require significant energy. Examples of covalent bonds involving nitrogen include the triple bond in nitrogen gas (N₂) and the single bonds in ammonia (NH₃). The ability to form strong triple bonds in N₂ leads to its inertness at room temperature.
-
Electronegativity: Nitrogen is a relatively electronegative element, meaning it has a strong tendency to attract electrons in a chemical bond. This electronegativity is a consequence of its relatively high nuclear charge and compact size, both of which attract electrons towards the nucleus. This electronegativity influences the polarity of the bonds it forms.
-
Oxidation States: Nitrogen exhibits a wide range of oxidation states, from -3 (as in ammonia, NH₃) to +5 (as in nitric acid, HNO₃). This versatility arises from its ability to gain or lose electrons, depending on the chemical environment.
Nitrogen's Role in Biological Systems
The five valence electrons of nitrogen are fundamental to its crucial role in biological systems:
-
Amino Acids and Proteins: Nitrogen is a key component of amino acids, the building blocks of proteins. The amino group (-NH₂) in amino acids contains nitrogen atoms covalently bonded to carbon and hydrogen atoms. The presence of nitrogen contributes to the diverse functionalities and structures of proteins, vital for biological processes.
-
Nucleic Acids (DNA and RNA): Nitrogen is also essential to the structure of nucleic acids, DNA and RNA, which carry genetic information. Nitrogenous bases, like adenine, guanine, cytosine, and thymine (or uracil in RNA), contain nitrogen atoms within their ring structures. These bases are crucial for the formation of the DNA double helix and RNA's various functionalities.
Nitrogen's Industrial Applications
The chemical reactivity of nitrogen, dictated by its five valence electrons, underlies its wide-ranging industrial applications:
-
Fertilizers: Nitrogen is a critical component of fertilizers, crucial for plant growth. Ammonia (NH₃), produced through the Haber-Bosch process, is a major source of nitrogen for fertilizers. This process utilizes nitrogen gas (N₂), highlighting the importance of the strong triple bond in N₂ and the energy input required to break it and facilitate its reaction.
-
Explosives: Certain nitrogen-containing compounds, such as nitroglycerin and TNT (trinitrotoluene), are highly explosive. The energy released during the decomposition of these molecules is directly related to the strength of nitrogen-oxygen bonds and their rearrangements.
-
Materials Science: Nitrogen is incorporated into various materials to modify their properties. For example, nitrogen doping can enhance the performance of semiconductors and other materials.
Comparing Nitrogen to Other Elements in Group 15
Nitrogen belongs to Group 15 (also known as Group VA or the pnictogens) of the periodic table. All elements in this group have five valence electrons. However, their properties vary considerably due to differences in atomic size and electronegativity. For example, phosphorus, arsenic, antimony, and bismuth, also have five valence electrons, but they show greater tendency to form covalent bonds with oxygen compared to nitrogen. Their lower electronegativity is a key factor influencing this variation in behavior.
Conclusion: The Central Role of Valence Electrons
The number of valence electrons profoundly impacts an element's chemical behavior and properties. Nitrogen, with its five valence electrons, exhibits a remarkable range of reactivity and applications. Its ability to form covalent bonds, its electronegativity, and its diverse oxidation states all stem directly from this fundamental aspect of its atomic structure. Understanding nitrogen's electronic configuration and its five valence electrons is crucial for comprehending its pivotal role in both biological systems and industrial processes. From the air we breathe to the fertilizers that support our food production, nitrogen's properties, driven by its five valence electrons, are essential for life and modern society. Further study into the nuances of bonding, reactivity, and electronic configuration of nitrogen and other elements continues to be a driving force in scientific innovation and technological advancement.
Latest Posts
Latest Posts
-
How Does Longer Contact Increase Dissolution
Mar 25, 2025
-
How Hot Is 35 Degrees Celsius
Mar 25, 2025
-
31 Degrees Celsius Is What Fahrenheit
Mar 25, 2025
-
14 To The Power Of 2
Mar 25, 2025
-
Is Kool Aid A Homogeneous Mixture
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Does N Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
