How Many Valence Electrons Does Pcl3 Have
Kalali
Mar 30, 2025 · 6 min read
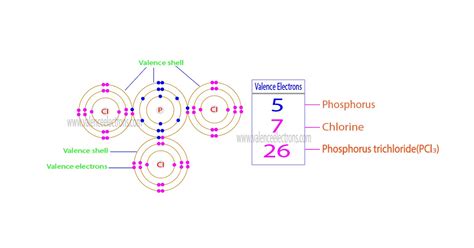
Table of Contents
How Many Valence Electrons Does PCl₃ Have? A Deep Dive into Phosphorus Trichloride
Determining the number of valence electrons in a molecule like phosphorus trichloride (PCl₃) is crucial for understanding its bonding, structure, and reactivity. This seemingly simple question opens the door to a fascinating exploration of fundamental chemistry principles. Let's delve into the details, exploring not just the answer but also the underlying concepts that lead us there.
Understanding Valence Electrons
Before we tackle PCl₃ specifically, let's establish a firm grasp on what valence electrons are. Valence electrons are the electrons located in the outermost shell (or energy level) of an atom. These are the electrons that participate in chemical bonding, determining an element's reactivity and the types of bonds it can form. They are the key players in the chemical game!
The number of valence electrons an atom possesses is determined by its position in the periodic table. Specifically, it's dictated by the atom's group number (for the main group elements). For instance, elements in Group 1 (alkali metals) have one valence electron, Group 2 (alkaline earth metals) have two, and so on. Things get a bit more nuanced for transition metals, but for main group elements, this rule provides a straightforward approach.
Phosphorus (P): Unveiling its Valence Electrons
Phosphorus, the central atom in PCl₃, resides in Group 15 (also known as Group VA) of the periodic table. This means a phosphorus atom has five valence electrons. These five electrons are available to participate in chemical bonding. Understanding this is the cornerstone of calculating the total valence electrons in PCl₃.
Chlorine (Cl): Contributing its Share
Chlorine (Cl), the other element in PCl₃, is located in Group 17 (Group VIIA), the halogens. This placement signifies that each chlorine atom possesses seven valence electrons. Since there are three chlorine atoms in PCl₃, the total contribution from chlorine is 7 electrons/atom * 3 atoms = 21 valence electrons.
Calculating the Total Valence Electrons in PCl₃
Now, we can combine the contributions from phosphorus and chlorine to determine the total number of valence electrons in the PCl₃ molecule:
- Phosphorus: 5 valence electrons
- Chlorine (3 atoms): 21 valence electrons
- Total: 5 + 21 = 26 valence electrons
Therefore, the phosphorus trichloride molecule (PCl₃) has a total of 26 valence electrons. This number is crucial for drawing the Lewis structure and predicting the molecule's geometry.
Drawing the Lewis Structure of PCl₃
The Lewis structure, also known as the electron dot structure, visually represents the arrangement of valence electrons in a molecule. Drawing this structure helps us visualize the bonding and understand the molecule's properties.
Here's how to construct the Lewis structure for PCl₃:
-
Central Atom: Place the phosphorus atom (P) in the center. Phosphorus is less electronegative than chlorine, making it the central atom.
-
Surrounding Atoms: Surround the phosphorus atom with the three chlorine atoms (Cl).
-
Single Bonds: Connect each chlorine atom to the phosphorus atom with a single bond (represented by a line). Each single bond consists of two electrons. This uses 6 electrons (3 bonds * 2 electrons/bond).
-
Remaining Electrons: Distribute the remaining valence electrons (26 - 6 = 20 electrons) as lone pairs around the atoms, starting with the outer atoms (chlorine) to fulfill their octets (8 electrons). Each chlorine atom needs 6 more electrons (7 valence electrons - 1 bond electron), requiring 18 electrons (3 chlorine atoms * 6 electrons/atom). This leaves 2 electrons.
-
Lone Pair on Phosphorus: Place the remaining 2 electrons as a lone pair on the phosphorus atom.
The completed Lewis structure shows phosphorus surrounded by three chlorine atoms, with three single bonds and one lone pair on phosphorus.
Understanding the Geometry of PCl₃
The Lewis structure provides the foundation for understanding the molecular geometry of PCl₃. The presence of the lone pair on the phosphorus atom significantly influences the shape. The VSEPR (Valence Shell Electron Pair Repulsion) theory helps predict the three-dimensional arrangement of atoms in a molecule based on electron pair repulsion.
According to VSEPR theory, the electron pairs (both bonding and non-bonding) around the central atom arrange themselves to minimize repulsion. In PCl₃, there are four electron pairs around the phosphorus atom: three bonding pairs (P-Cl bonds) and one lone pair. This arrangement leads to a tetrahedral electron-pair geometry.
However, the molecular geometry, which considers only the positions of the atoms, is trigonal pyramidal. The lone pair occupies space, pushing the three chlorine atoms slightly closer together. This results in a pyramidal shape rather than a perfectly flat trigonal planar structure.
PCl₃'s Polarity
The asymmetrical distribution of charge in PCl₃ due to its trigonal pyramidal geometry and the difference in electronegativity between phosphorus and chlorine leads to a polar molecule. The chlorine atoms are more electronegative than phosphorus, meaning they attract the shared electrons in the P-Cl bonds more strongly. This creates a net dipole moment, making PCl₃ a polar molecule.
Applications of PCl₃
Phosphorus trichloride is a versatile chemical compound with significant applications in various industries. It serves as a crucial intermediate in the production of organophosphorus compounds. These compounds are used extensively in several applications including:
-
Pesticides: PCl₃ is a precursor in the synthesis of various organophosphate insecticides.
-
Plastics and Polymers: Organophosphorus compounds derived from PCl₃ find use in flame retardants and in the production of some types of polymers.
-
Pharmaceuticals: Some organophosphorus compounds are used in the pharmaceutical industry, either as active ingredients or in the synthesis of other drugs.
-
Other applications: PCl₃ can also be used as a chlorinating agent.
Safety Precautions with PCl₃
It's crucial to emphasize the importance of safety when handling PCl₃. It is a highly reactive and toxic compound. Direct contact with PCl₃ can cause severe burns to the skin and eyes. Inhalation can lead to serious respiratory problems. Therefore, appropriate safety measures, including personal protective equipment (PPE), and well-ventilated areas are absolutely necessary when working with this compound.
Conclusion: Beyond the Valence Electrons
While initially, the question seemed simple—how many valence electrons does PCl₃ have?—we've uncovered a rich understanding of chemical bonding, molecular geometry, and the properties of a vital chemical compound. The 26 valence electrons are not merely a number; they are the fundamental building blocks that determine PCl₃'s structure, reactivity, and applications. This exploration demonstrates how a seemingly simple calculation can open doors to a deep appreciation of chemical principles and their real-world impact. The journey from counting valence electrons to understanding the properties and applications of PCl₃ exemplifies the beauty and power of chemical inquiry. The interplay between theory (VSEPR, Lewis Structures) and practical applications underlines the significance of mastering fundamental chemical concepts.
Latest Posts
Latest Posts
-
What Percentage Is 35 Out Of 50
Apr 01, 2025
-
How To Make A Velocity Vs Time Graph
Apr 01, 2025
-
Is The Atlantic Colder Than The Pacific
Apr 01, 2025
-
What Is The Warmest Part Of The Day
Apr 01, 2025
-
48 Oz Equals How Many Cups
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Does Pcl3 Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
