How Was Bohr's Atomic Model Different From Rutherford's Atomic Model
Kalali
Mar 23, 2025 · 6 min read
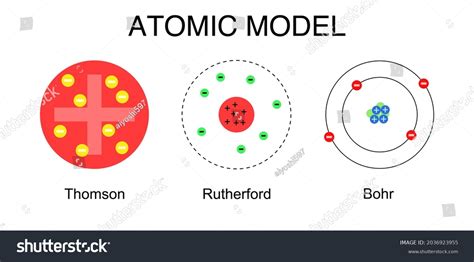
Table of Contents
How Was Bohr's Atomic Model Different from Rutherford's Atomic Model?
The development of the atomic model is a fascinating journey through the history of science, marked by groundbreaking discoveries and paradigm shifts. Two pivotal figures in this journey were Ernest Rutherford and Niels Bohr, whose models, while building upon each other, represented significant leaps forward in our understanding of the atom. This article delves into the key differences between Rutherford's and Bohr's atomic models, highlighting their strengths, limitations, and the lasting impact they had on the field of atomic physics.
Rutherford's Nuclear Model: A Revolutionary Leap
Before diving into the differences, let's briefly recap Rutherford's groundbreaking nuclear model, proposed in 1911. Rutherford's famous gold foil experiment demonstrated that the atom was not a solid, indivisible sphere as previously believed (the plum pudding model). Instead, his experiment revealed a surprisingly simple yet revolutionary picture:
Key Features of Rutherford's Model:
- Mostly Empty Space: The atom is mostly empty space. The alpha particles in Rutherford's experiment passed straight through the gold foil, indicating that there was little to obstruct their path.
- Dense, Positively Charged Nucleus: The positive charge of the atom is concentrated in a tiny, dense region at the center called the nucleus. This nucleus is responsible for most of the atom's mass.
- Electrons Orbiting the Nucleus: Negatively charged electrons orbit the nucleus at a relatively large distance. The electrostatic attraction between the positive nucleus and negative electrons holds the atom together.
Limitations of Rutherford's Model:
Despite its revolutionary nature, Rutherford's model suffered from significant limitations:
- Classical Physics Failure: It violated the principles of classical electromagnetism. According to classical physics, an orbiting electron should constantly emit electromagnetic radiation, losing energy and spiraling into the nucleus, causing the atom to collapse. This clearly didn't happen, suggesting a fundamental flaw in the model.
- No Explanation for Atomic Spectra: Rutherford's model failed to explain the discrete spectral lines observed when atoms emit light. Classical physics predicted a continuous spectrum, not the sharp, specific wavelengths actually observed.
- Electron Orbits Not Defined: The model did not specify the arrangement or energy levels of the electrons orbiting the nucleus.
Bohr's Atomic Model: Addressing the Shortcomings
Niels Bohr, building upon Rutherford's work, proposed his own atomic model in 1913, addressing many of the shortcomings of Rutherford's model by incorporating concepts from quantum theory.
Key Features of Bohr's Model:
- Quantized Energy Levels: Bohr's most significant contribution was the introduction of quantized energy levels. He postulated that electrons can only exist in specific, discrete energy levels or orbits around the nucleus. These energy levels are quantized, meaning they can only have certain specific values.
- Stationary Orbits: While orbiting the nucleus, electrons do not emit radiation as long as they remain in their quantized energy levels. These orbits are referred to as stationary states.
- Energy Absorption and Emission: Electrons can jump between energy levels by absorbing or emitting energy in the form of photons (light). The energy of the photon corresponds to the difference in energy between the two levels.
- Specific Spectral Lines: The discrete spectral lines observed in atomic spectra are explained by the transitions of electrons between these quantized energy levels. Each spectral line corresponds to a specific energy difference, and hence a specific wavelength of light.
Improvements over Rutherford's Model:
Bohr's model successfully explained several phenomena that Rutherford's model could not:
- Stability of the Atom: By introducing quantized energy levels, Bohr's model addressed the stability problem. Electrons in stationary states do not radiate energy, preventing the atom from collapsing.
- Explanation of Atomic Spectra: The model correctly predicted the discrete spectral lines observed in the hydrogen atom spectrum, providing a strong experimental validation. The specific wavelengths of light emitted corresponded precisely to the energy differences between electron energy levels.
- Predictive Power: Bohr's model provided a framework for predicting the wavelengths of spectral lines for other elements, although with less accuracy than for hydrogen.
Limitations of Bohr's Model:
Despite its successes, Bohr's model also faced limitations:
- Only Applicable to Hydrogen: It worked well for hydrogen, which has only one electron, but its predictive power was limited when applied to atoms with multiple electrons. The interactions between multiple electrons were too complex to be accurately described by the model.
- Classical and Quantum Mechanics Mix: It was a hybrid model, combining classical mechanics (planetary electron orbits) with quantum concepts (quantized energy levels). This mixture represented an uneasy compromise.
- Inability to Explain Fine Structure: The model could not account for the fine structure of spectral lines – the slight splitting of spectral lines observed under high resolution.
- No Explanation for Chemical Bonding: It failed to explain the nature of chemical bonding, which is crucial for understanding the behavior of molecules.
The Transition to Quantum Mechanics
Bohr's model, while a significant improvement over Rutherford's, was ultimately superseded by the development of quantum mechanics in the 1920s. The new quantum mechanical model, based on the work of Schrödinger, Heisenberg, and others, provided a more complete and accurate description of the atom.
Key Differences between Bohr's Model and Quantum Mechanics:
- Probability vs. Orbits: Quantum mechanics replaces the concept of definite electron orbits with probability distributions. Electrons are described by wave functions, which give the probability of finding an electron at a particular location.
- Orbitals vs. Orbits: Electrons occupy orbitals, regions of space where there is a high probability of finding the electron, instead of following well-defined orbits.
- Heisenberg Uncertainty Principle: Quantum mechanics incorporates the Heisenberg Uncertainty Principle, which states that it is impossible to simultaneously know both the position and momentum of an electron with perfect accuracy.
- More Accurate Predictions: Quantum mechanics provides far more accurate predictions for atomic spectra and other atomic properties than Bohr's model, especially for atoms with multiple electrons.
Conclusion: A Legacy of Progress
Both Rutherford's and Bohr's models were crucial milestones in the development of our understanding of the atom. Rutherford's model revolutionized our perception of atomic structure by introducing the concept of the nucleus. Bohr's model, while possessing limitations, significantly advanced our understanding by introducing the concept of quantized energy levels and explaining atomic spectra. Although superseded by quantum mechanics, Bohr's model served as a bridge between classical physics and quantum mechanics, paving the way for the more sophisticated models that followed. The legacies of Rutherford and Bohr remain significant in the history of science, underscoring the iterative and progressive nature of scientific discovery. Their contributions continue to inform and inspire our understanding of the fundamental building blocks of matter. The journey from a simple plum pudding model to the complex quantum mechanical description of the atom represents a triumph of human ingenuity and persistent investigation into the mysteries of the universe.
Latest Posts
Latest Posts
-
Compare And Contrast Young Adulthood With Middle Adulthood
Mar 25, 2025
-
What Do Peppered Moths Do During The Winter
Mar 25, 2025
-
Packages Proteins For Transport Out Of The Cell
Mar 25, 2025
-
How Many Feet Is 101 Inches
Mar 25, 2025
-
How Many Feet Is 150 In
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about How Was Bohr's Atomic Model Different From Rutherford's Atomic Model . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
