If Two Covalently Bonded Atoms Are Identical The Bond Is
Kalali
Mar 21, 2025 · 6 min read
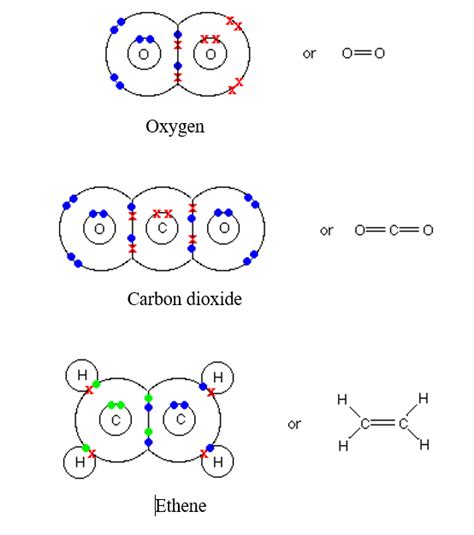
Table of Contents
If Two Covalently Bonded Atoms Are Identical, the Bond Is… Nonpolar Covalent!
When two atoms share electrons to form a chemical bond, it's called a covalent bond. But the nature of that bond can vary depending on the atoms involved. A crucial factor determining bond character is the electronegativity of the atoms. If the two covalently bonded atoms are identical, the bond is a nonpolar covalent bond. Let's delve into the details of this crucial concept in chemistry.
Understanding Electronegativity
Before diving into the specifics of identical atoms forming covalent bonds, it's crucial to grasp the concept of electronegativity. Electronegativity is a measure of an atom's ability to attract electrons towards itself in a chemical bond. Atoms with high electronegativity strongly pull electrons towards them, while atoms with low electronegativity hold onto electrons less tightly. The Pauling scale is commonly used to represent electronegativity values, with fluorine (F) having the highest value of 4.0.
The Electronegativity Difference and Bond Type
The difference in electronegativity between two bonded atoms dictates the nature of the bond:
-
Nonpolar Covalent Bond: When the electronegativity difference between two atoms is zero or very small (generally less than 0.5 on the Pauling scale), the electrons are shared almost equally between the atoms. This results in a nonpolar covalent bond. This is precisely the case when the two atoms are identical.
-
Polar Covalent Bond: If the electronegativity difference is significant (generally between 0.5 and 1.7), the electrons are shared unequally. The more electronegative atom attracts the electrons more strongly, resulting in a polar covalent bond. This creates a slightly negative charge (δ-) on the more electronegative atom and a slightly positive charge (δ+) on the less electronegative atom.
-
Ionic Bond: When the electronegativity difference is very large (generally greater than 1.7), the more electronegative atom essentially steals the electron(s) from the less electronegative atom. This leads to the formation of ions – positively charged cations and negatively charged anions – and the resulting bond is called an ionic bond.
Identical Atoms and Nonpolar Covalent Bonds
The beauty of a covalent bond between identical atoms lies in its symmetry. Since the atoms are identical, they possess the same electronegativity. Therefore, there's no difference in their ability to attract the shared electrons. The electrons are shared equally, resulting in a perfectly balanced distribution of charge. This perfect balance is the hallmark of a nonpolar covalent bond.
Examples of Nonpolar Covalent Bonds
Numerous examples illustrate this principle:
-
Hydrogen (H₂): Two hydrogen atoms share a pair of electrons equally to form a diatomic hydrogen molecule (H₂). The bond is purely nonpolar because the electronegativity difference is zero.
-
Oxygen (O₂): Two oxygen atoms share electrons to form a diatomic oxygen molecule (O₂), forming a nonpolar double bond. Again, the electronegativity difference is zero.
-
Nitrogen (N₂): Two nitrogen atoms form a diatomic nitrogen molecule (N₂) via a triple bond. The electron sharing remains perfectly balanced, resulting in a nonpolar covalent bond.
-
Chlorine (Cl₂): Similar to the above examples, two chlorine atoms form a diatomic chlorine molecule (Cl₂) through a nonpolar single bond.
-
Other Diatomic Molecules: All diatomic molecules composed of identical atoms, such as bromine (Br₂), iodine (I₂), and fluorine (F₂), exhibit nonpolar covalent bonding.
Delving Deeper into Bond Properties
The nonpolar nature of a covalent bond between identical atoms affects several crucial properties:
1. Bond Polarity and Dipole Moment
A dipole moment is a measure of the separation of positive and negative charges within a molecule. In nonpolar covalent bonds, the dipole moment is zero. This is because the electron distribution is symmetrical, leading to no net charge separation. In contrast, polar molecules possess a non-zero dipole moment.
2. Boiling and Melting Points
Generally, nonpolar molecules have lower boiling and melting points compared to polar molecules. This is because the intermolecular forces (forces between molecules) in nonpolar substances are weaker (primarily London dispersion forces). Weaker intermolecular forces require less energy to overcome, leading to lower melting and boiling points.
3. Solubility
Nonpolar molecules tend to be soluble in nonpolar solvents but insoluble in polar solvents. This is governed by the "like dissolves like" principle. Nonpolar substances interact favorably with other nonpolar substances, but not with polar substances.
4. Reactivity
The reactivity of a molecule is influenced by its bond polarity. Nonpolar molecules generally exhibit lower reactivity than polar molecules because they have a more stable, balanced electron distribution. However, this is a generalization, and other factors, such as bond strength, also play a significant role.
Beyond Diatomic Molecules: Identical Atoms in Larger Molecules
While the examples above primarily focus on diatomic molecules, the principle extends to larger molecules. Within a larger molecule, if two identical atoms are covalently bonded, the bond between them will be nonpolar. For example, consider ethane (C₂H₆): The carbon-carbon bond (C-C) is nonpolar because the two carbon atoms have identical electronegativity.
Exceptions and Nuances
While the concept is straightforward, there are subtle nuances to consider:
-
Bonding in Polyatomic Ions: In polyatomic ions, such as the carbonate ion (CO₃²⁻), the bonds might not be perfectly nonpolar even if identical atoms are involved due to resonance and charge distribution effects. While the carbon-oxygen bonds are largely covalent, the overall charge distribution makes them polar.
-
Influence of Molecular Geometry: Molecular geometry plays a crucial role in determining overall molecular polarity. Even if individual bonds are nonpolar, the overall molecule can be polar due to the asymmetrical arrangement of atoms. For instance, consider carbon dioxide (CO₂). Each carbon-oxygen bond is polar. However, the linear geometry of the molecule ensures that the bond dipoles cancel out, resulting in a nonpolar molecule. This contrasts with water (H₂O), where the bent geometry prevents the cancellation of bond dipoles, yielding a polar molecule.
-
Inductive Effects: In larger molecules, the presence of electronegative atoms elsewhere in the molecule can influence the electron distribution around seemingly identical bonds, making even bonds between identical atoms slightly polar. These inductive effects are relatively small but can impact bond character to a slight extent.
Conclusion
In summary, when two covalently bonded atoms are identical, the bond is nonpolar covalent. This fundamental concept forms the basis for understanding a wide range of chemical properties, including bond polarity, boiling points, melting points, solubility, and reactivity. While diatomic molecules provide simple examples, this principle extends to larger molecules, with nuances arising from factors such as resonance, molecular geometry, and inductive effects. Understanding the concept of electronegativity and its relationship to bond type is essential for grasping the diverse world of chemical bonding.
Latest Posts
Latest Posts
-
How Many Feet In 35 Inches
Mar 22, 2025
-
How Many Feet Are In 90 Inches
Mar 22, 2025
-
The Ratio Of Atoms In Hcl Is
Mar 22, 2025
-
4 Of 18 Is What Percent
Mar 22, 2025
-
What Percent Is 8 Of 12
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about If Two Covalently Bonded Atoms Are Identical The Bond Is . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
