In Osmosis Water Always Moves Toward The
Kalali
Mar 26, 2025 · 6 min read
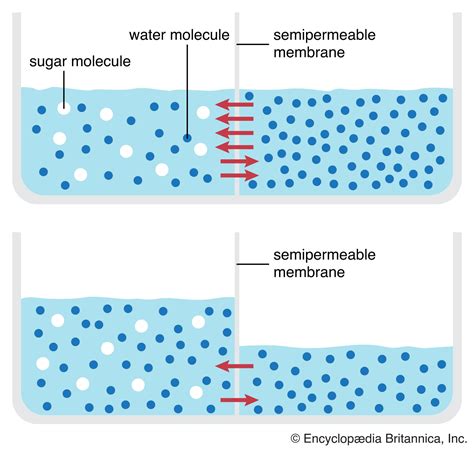
Table of Contents
In Osmosis, Water Always Moves Toward the Higher Solute Concentration: A Deep Dive
Osmosis, a fundamental process in biology and chemistry, governs the movement of water across selectively permeable membranes. Understanding this process is crucial for comprehending various biological phenomena, from plant turgor pressure to the function of kidneys. A common misconception is that water moves towards areas of higher water concentration. However, the reality is more nuanced: in osmosis, water always moves toward the area with a higher solute concentration. This movement continues until equilibrium is reached, meaning the solute concentration is equal on both sides of the membrane. This article delves into the intricacies of osmosis, explaining why water behaves this way and its significant implications.
Understanding Osmosis: A Simple Analogy
Imagine two compartments separated by a selectively permeable membrane—a barrier that allows some substances to pass through but restricts others. One compartment contains a high concentration of sugar dissolved in water (a hypertonic solution), while the other contains pure water (a hypotonic solution). The membrane only allows water molecules to pass through.
What happens? The water molecules, driven by their inherent kinetic energy, constantly move randomly. However, the net movement of water is from the hypotonic solution (pure water) to the hypertonic solution (sugar water). Why? Because the sugar molecules in the hypertonic solution prevent some water molecules from crossing back into the hypotonic solution. This creates an imbalance, and the net movement continues until the water concentration becomes equal on both sides of the membrane. This is osmosis.
The Role of Water Potential
To understand the direction of water movement more precisely, we need to introduce the concept of water potential. Water potential is the potential energy of water per unit volume, relative to pure water at atmospheric pressure and temperature. It's expressed in units of pressure (e.g., megapascals, MPa).
- Pure water has the highest water potential (0 MPa).
- Adding solutes to water lowers its water potential, making it a negative value. The more solute added, the more negative the water potential becomes.
During osmosis, water always moves from an area of higher water potential (less negative) to an area of lower water potential (more negative). Since adding solutes lowers water potential, water moves towards the area with the higher solute concentration, ultimately aiming to equalize the water potential across the membrane.
Factors Affecting Osmosis
Several factors influence the rate and direction of osmosis:
1. Solute Concentration Gradient: The Driving Force
The steeper the solute concentration gradient (the bigger the difference in solute concentration between the two compartments), the faster the rate of osmosis. A larger difference means a greater driving force for water to move toward the hypertonic solution.
2. Membrane Permeability: Selective Passage
The selectively permeable membrane plays a crucial role. The membrane's properties determine which molecules can pass through and how easily. A more permeable membrane will allow for a faster rate of osmosis. The type of membrane also determines the specifics of the osmotic pressure and effects.
3. Temperature: Kinetic Energy
Higher temperatures increase the kinetic energy of water molecules, leading to a faster rate of osmosis. Increased molecular movement facilitates a more rapid equalization of water potential.
4. Pressure: Opposing Force
Applying pressure to the hypertonic solution can counteract osmosis. By increasing the pressure, you essentially force water to move against the concentration gradient. This principle is used in reverse osmosis, a technique for purifying water.
Osmosis in Biological Systems: Real-World Examples
Osmosis is not just a laboratory phenomenon; it's a fundamental process governing life itself.
1. Plant Cells: Turgor Pressure
Plant cells rely on osmosis for maintaining their turgor pressure. When a plant cell is placed in a hypotonic solution, water moves into the cell, causing it to swell. This creates turgor pressure, pushing the cell membrane against the rigid cell wall, giving the plant its firmness and structure. Conversely, in a hypertonic solution, water leaves the cell, causing it to plasmolyze (shrink).
2. Animal Cells: Maintaining Cell Shape
Animal cells don't have a rigid cell wall, making them more susceptible to changes in osmotic pressure. In a hypotonic solution, animal cells can swell and even burst (lyse) due to excessive water uptake. In a hypertonic solution, they shrink (crenate) as water leaves the cell. The maintenance of proper osmotic balance is crucial for animal cell survival.
3. Kidney Function: Water Regulation
The kidneys play a vital role in regulating water balance in the body. Through processes like filtration and reabsorption, the kidneys control the amount of water excreted in urine, maintaining the proper osmotic balance in the blood. This is essential for preventing dehydration or overhydration.
4. Absorption of Nutrients: Intestinal Tract
Osmosis plays a crucial part in nutrient absorption in the digestive system. Water moves across the intestinal lining, facilitating the absorption of digested nutrients into the bloodstream.
Osmotic Pressure: A Quantifiable Force
Osmotic pressure is the pressure required to prevent the net flow of water across a selectively permeable membrane. It's directly proportional to the solute concentration. The higher the solute concentration, the higher the osmotic pressure.
Reverse Osmosis: A Technological Application
Reverse osmosis is a technology that uses pressure to force water across a semipermeable membrane against its natural osmotic flow. This process is used for water purification by removing dissolved salts, minerals, and other impurities from water.
Practical Applications and Further Research
Understanding osmosis has led to numerous practical applications, including:
- Water purification: Reverse osmosis is widely used for desalination and producing purified water for drinking and industrial purposes.
- Medical treatments: Osmosis is crucial in dialysis, a treatment for kidney failure, where waste products are removed from the blood.
- Agriculture: Understanding osmosis helps farmers manage irrigation and fertilization effectively.
- Food preservation: Osmosis is used in some food preservation techniques to control water content and microbial growth.
Further research into osmosis continues to yield valuable insights into diverse fields. Studies are focused on:
- Developing new membrane materials with enhanced permeability and selectivity for various applications.
- Investigating the role of osmosis in various physiological processes, including those related to cell signaling and disease.
- Exploring the application of osmosis in energy production and environmental remediation.
Conclusion
In conclusion, osmosis, governed by the principle of water moving from a higher water potential to a lower water potential, is a fundamental process with far-reaching implications. Understanding the factors influencing osmosis and its role in biological systems is crucial for advancing our knowledge in various scientific fields and solving practical challenges. The constant movement of water towards the area of higher solute concentration is the driving force behind many essential life processes, from maintaining cell shape to regulating blood pressure. Continued research in this field promises even more exciting discoveries and applications in the years to come.
Latest Posts
Latest Posts
-
Does Hot Air Rise Or Sink
Mar 29, 2025
-
Cual Es El Planeta Mas Cercano Al Sol
Mar 29, 2025
-
Which Polymers Are Composed Of Amino Acids
Mar 29, 2025
-
Convert 1 1 4 Inch To Mm
Mar 29, 2025
-
What Is 30 Off Of 50
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about In Osmosis Water Always Moves Toward The . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
