In The Modern Periodic Table How Are The Elements Arranged
Kalali
Mar 15, 2025 · 5 min read
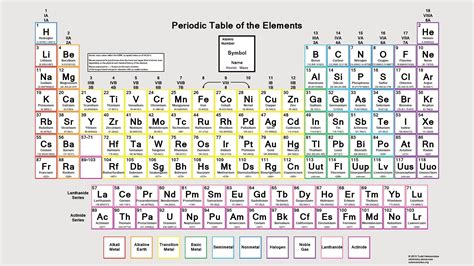
Table of Contents
In the Modern Periodic Table: How Are the Elements Arranged?
The modern periodic table, a cornerstone of chemistry, isn't just a random collection of elements. Its elegant structure reflects the fundamental properties of matter and allows us to predict the behavior of elements with remarkable accuracy. Understanding how the elements are arranged is key to grasping the principles of chemistry and unlocking the secrets of the universe. This article will delve deep into the organization of the modern periodic table, explaining the underlying principles and the significance of its arrangement.
The Foundation: Atomic Number and Electron Configuration
The fundamental organizing principle of the modern periodic table is the atomic number. Each element is uniquely identified by its atomic number, which represents the number of protons in its nucleus. This number dictates the element's identity and largely determines its chemical properties. Crucially, the number of protons also dictates the number of electrons in a neutral atom, shaping its electron configuration.
Electron Shells and Subshells
Electrons aren't randomly scattered around the nucleus. They occupy specific energy levels, or shells, arranged in increasing distance from the nucleus. These shells are further subdivided into subshells (s, p, d, and f), each capable of holding a specific number of electrons. The filling of these subshells follows the Aufbau principle, which states that electrons fill the lowest energy levels first. This principle, along with the Pauli exclusion principle (which limits the number of electrons per orbital) and Hund's rule (which governs electron distribution within subshells), determines the electron configuration of each element.
Understanding electron configuration is crucial because it directly influences an element's chemical behavior. Electrons in the outermost shell, known as valence electrons, are primarily responsible for chemical bonding and reactivity. Elements with similar valence electron configurations exhibit similar chemical properties.
The Periodic Table's Structure: Periods and Groups
The modern periodic table arranges elements in a grid-like format, with elements organized into periods (rows) and groups (columns).
Periods: Reflecting Energy Levels
Each period represents a principal energy level or shell. The first period, containing only hydrogen and helium, corresponds to the filling of the first electron shell (n=1). The second period (lithium to neon) fills the second shell (n=2), and so on. As you move down the table, you are adding another shell to the electron configuration. The length of each period varies as it corresponds to the number of electrons that can occupy the subshells being filled.
Groups: Reflecting Valence Electrons
Groups, or families, are the vertical columns in the periodic table. Elements within the same group share similar chemical properties because they possess the same number of valence electrons. This similarity in valence electron configuration leads to similar bonding behaviors and reactivity.
Some key groups include:
- Group 1 (Alkali Metals): Highly reactive metals with one valence electron.
- Group 2 (Alkaline Earth Metals): Reactive metals with two valence electrons.
- Group 17 (Halogens): Highly reactive nonmetals with seven valence electrons.
- Group 18 (Noble Gases): Inert gases with a full valence shell (eight electrons, except for helium with two), making them exceptionally stable and unreactive.
- Transition Metals (Groups 3-12): These elements are characterized by the filling of d orbitals, leading to a wider range of oxidation states and complex chemical behaviors.
- Lanthanides and Actinides: These elements are placed separately at the bottom of the table. They are characterized by the filling of the 4f and 5f orbitals, respectively.
The Significance of the Arrangement: Predicting Properties
The periodic table's arrangement isn't merely a convenient way to organize elements; it's a powerful tool for predicting their properties. By understanding an element's position on the table, we can infer its:
- Metallic Character: Metallic character generally increases as you move down a group and decreases as you move across a period from left to right.
- Electronegativity: Electronegativity, the ability of an atom to attract electrons in a chemical bond, generally increases across a period and decreases down a group.
- Ionization Energy: The energy required to remove an electron from an atom, generally increases across a period and decreases down a group.
- Atomic Radius: Atomic size generally increases down a group and decreases across a period.
- Reactivity: The reactivity of elements is directly related to their electron configuration, particularly the number of valence electrons.
Beyond the Basics: Variations and Exceptions
While the periodic table provides a remarkable framework for understanding elemental properties, there are some exceptions and nuances:
- Transition Metals: The transition metals exhibit a broader range of oxidation states and complex chemical behaviors due to the variable filling of their d orbitals.
- Lanthanides and Actinides: The f-block elements display unique properties related to the filling of their f orbitals.
- Anomalous Electron Configurations: Some elements deviate slightly from the expected electron configurations predicted by the Aufbau principle due to the subtle energy differences between subshells.
The Ongoing Evolution of the Periodic Table
The periodic table isn't static. As new elements are synthesized and discovered, our understanding of their properties deepens, leading to refinements in our understanding of periodic trends. The ongoing research in nuclear chemistry and materials science continues to push the boundaries of our knowledge and reshape our understanding of the periodic table.
Conclusion: A Powerful Tool for Understanding Matter
The modern periodic table's arrangement, based on atomic number and electron configuration, is a testament to the elegance and power of scientific understanding. Its systematic organization provides a framework for predicting the properties of elements, understanding their chemical behavior, and driving advancements in various fields, including materials science, medicine, and technology. Its inherent structure reflects fundamental principles of atomic structure, forming the bedrock of chemistry and our comprehension of the material world. The periodic table is more than just a chart; it's a powerful tool that continues to shape our understanding of the universe and its constituent elements. Understanding its organization is essential for anyone seeking to delve deeper into the fascinating world of chemistry.
Latest Posts
Latest Posts
-
Is Reacts With Air A Physical Or Chemical Property
Mar 15, 2025
-
Is Mg A Metal Nonmetal Or Metalloid
Mar 15, 2025
-
What Percent Of 8 Is 2
Mar 15, 2025
-
157 Inches Is How Many Feet
Mar 15, 2025
-
What Does Water And Lava Make
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about In The Modern Periodic Table How Are The Elements Arranged . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
