Is Mg A Metal Nonmetal Or Metalloid
Kalali
Mar 15, 2025 · 5 min read
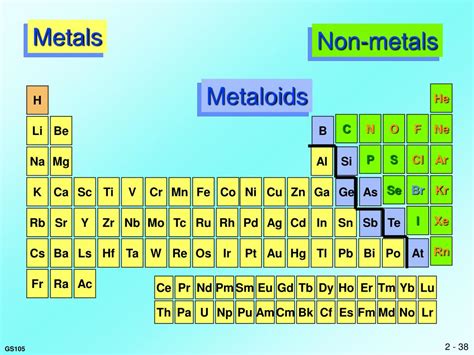
Table of Contents
Is Mg a Metal, Nonmetal, or Metalloid? A Deep Dive into Magnesium's Properties
Magnesium (Mg), element number 12 on the periodic table, is unequivocally a metal. This seemingly simple answer belies a rich understanding of its chemical and physical properties, which firmly place it within the metallic category. This article will delve deep into the characteristics that define magnesium as a metal, differentiating it from nonmetals and metalloids, and exploring its unique applications stemming from these properties.
Understanding the Classification of Elements
Before we definitively label magnesium, let's briefly review the fundamental differences between metals, nonmetals, and metalloids. This classification system helps us understand the behavior and reactivity of elements.
Metals: The Hallmarks of Conductivity and Malleability
Metals are characterized by their excellent electrical conductivity, thermal conductivity, malleability (ability to be hammered into sheets), ductility (ability to be drawn into wires), and luster (shiny appearance). They typically form positive ions (cations) by losing electrons. Their atomic structures involve a "sea" of delocalized electrons, accounting for their conductivity properties. Most metals are solid at room temperature, with the exception of mercury.
Nonmetals: Insulators and Diverse Reactivity
Nonmetals, on the other hand, are generally poor conductors of heat and electricity. They are often brittle and lack the characteristic luster of metals. Their reactivity varies greatly; some are highly reactive (like fluorine), while others are relatively inert (like noble gases). They tend to gain electrons to form negative ions (anions) and exist in various states at room temperature – solid, liquid, or gas.
Metalloids: Bridging the Gap
Metalloids, also known as semimetals, possess properties intermediate between metals and nonmetals. Their conductivity can be influenced by factors like temperature or pressure, leading to applications in semiconductors. They exhibit a mix of metallic and nonmetallic characteristics, making their categorization less straightforward. Examples include silicon, germanium, and arsenic.
Magnesium: A Definitive Metal
Magnesium unequivocally displays the hallmarks of a metal. Let's examine its properties in detail:
1. Excellent Electrical and Thermal Conductivity
Magnesium is a good conductor of both electricity and heat. This property stems from its atomic structure, where valence electrons are loosely held and can move freely, facilitating the transfer of charge and thermal energy. This is a key characteristic differentiating it from nonmetals, which lack this free electron mobility. Its conductivity, while not as high as that of copper or silver, is sufficient for various applications where lightweight and strong materials are preferred.
2. Malleability and Ductility: Shaping Magnesium
Magnesium's malleability and ductility allow it to be readily shaped into various forms, including sheets, wires, and complex shapes through processes like rolling, extrusion, and forging. This ease of manipulation, combined with its lightweight nature, makes it an ideal material for numerous engineering applications. Nonmetals, in contrast, are typically brittle and fracture easily under stress.
3. Metallic Luster: The Characteristic Shine
Magnesium possesses a characteristic metallic luster, a silvery-white shine that reflects light. This is a visual indicator of its metallic nature, absent in most nonmetals. The luster can be affected by oxidation, resulting in a dulling of the surface, but the underlying metallic structure remains.
4. Formation of Positive Ions (Cations): Reactivity Explained
Magnesium readily loses its two valence electrons to form a +2 cation (Mg²⁺). This tendency to lose electrons is a defining characteristic of metals. This ionic behavior is responsible for many of magnesium's chemical reactions, such as its reaction with acids to produce hydrogen gas. This contrasts sharply with nonmetals, which typically gain electrons to achieve a stable electron configuration.
5. Crystalline Structure: Orderly Arrangement of Atoms
Like most metals, magnesium exhibits a crystalline structure, a highly ordered arrangement of atoms in a three-dimensional lattice. This arrangement contributes to its strength and other mechanical properties. The regular pattern of atoms and the delocalized electrons account for many of its physical properties.
Magnesium's Applications: A Testament to its Metallic Nature
The diverse applications of magnesium are a direct consequence of its unique metallic properties. Let's look at some examples:
1. Lightweight Alloys: Aerospace and Automotive Industries
Magnesium's low density, combined with its strength and ease of fabrication, makes it highly desirable in the aerospace and automotive industries for creating lightweight yet strong components. These alloys significantly reduce the weight of vehicles and aircraft, improving fuel efficiency and performance. The ability to form strong alloys is a quintessential metallic characteristic.
2. Electronics: A Conductive Component
Magnesium's electrical conductivity finds applications in electronic components. While not as conductive as copper, its lightweight nature and other desirable properties make it suitable for specific applications where weight is a critical factor.
3. Biomedical Applications: Biocompatible and Biodegradable
Magnesium's biocompatibility and biodegradability have led to its use in biodegradable implants and stents. Its gradual degradation in the body eliminates the need for a second surgery to remove the implant.
4. Chemical Industry: Reducing Agent and Catalyst
Magnesium's reactivity allows it to serve as a powerful reducing agent in various chemical processes. It's also used as a catalyst in certain reactions, showcasing its versatility in chemical applications.
Distinguishing Magnesium from Metalloids: A Clear Divide
The properties of magnesium clearly distinguish it from metalloids. While metalloids exhibit a blend of metallic and nonmetallic characteristics, magnesium consistently displays the characteristics of a metal. Metalloids often have variable electrical conductivity, depending on factors like temperature and purity, whereas magnesium's conductivity is relatively consistent and characteristic of a metal. Metalloids are often semiconductors, a property not exhibited by magnesium.
Conclusion: Magnesium – A True Metal
In conclusion, magnesium's properties leave no room for doubt: it is unequivocally a metal. Its excellent electrical and thermal conductivity, malleability, ductility, metallic luster, formation of positive ions, and crystalline structure all firmly place it within the metallic category. Its wide range of applications, from lightweight alloys to biodegradable implants, further underscores its importance as a valuable metallic element. The distinction between metals, nonmetals, and metalloids is crucial for understanding the diverse behavior and applications of elements, and in the case of magnesium, the classification is clear and unambiguous. Its metallic properties are fundamental to its usefulness and have shaped its prominent role in various industries.
Latest Posts
Latest Posts
-
How Many Feet Is 26 In
Mar 17, 2025
-
What Is 6 Out Of 20 As A Percentage
Mar 17, 2025
-
What Is Melting Point Of Glass
Mar 17, 2025
-
Cuanto Es El 30 Por Ciento De 500
Mar 17, 2025
-
How Many Valence Electrons Do The Halogens Possess
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Is Mg A Metal Nonmetal Or Metalloid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
