Indicate Which Reactions Are Redox Reactions.
Kalali
Mar 27, 2025 · 6 min read
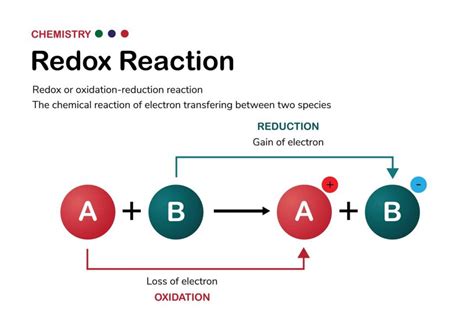
Table of Contents
Identifying Redox Reactions: A Comprehensive Guide
Redox reactions, short for reduction-oxidation reactions, are fundamental chemical processes that underpin a vast array of natural phenomena and industrial applications. Understanding how to identify these reactions is crucial for anyone studying chemistry, from high school students to seasoned researchers. This comprehensive guide will equip you with the knowledge and tools to confidently determine whether a given reaction is a redox reaction. We'll explore the core concepts, provide clear examples, and offer strategies for tackling complex scenarios.
Understanding the Basics: Oxidation and Reduction
At the heart of every redox reaction lies the transfer of electrons between chemical species. Oxidation refers to the loss of electrons, while reduction signifies the gain of electrons. These two processes are always coupled; you cannot have one without the other. Remember the mnemonic device OIL RIG: Oxidation Is Loss, Reduction Is Gain.
Let's illustrate with a simple example:
2Na + Cl₂ → 2NaCl
In this reaction, sodium (Na) atoms lose an electron each to become positively charged sodium ions (Na⁺). This is oxidation. Simultaneously, chlorine (Cl₂) molecules gain electrons, with each chlorine atom gaining one electron to become negatively charged chloride ions (Cl⁻). This is reduction. The overall reaction is a redox reaction because both oxidation and reduction occur simultaneously.
Key Indicators of Redox Reactions
Identifying redox reactions often involves looking for specific changes in the oxidation states of elements involved. The oxidation state (or oxidation number) represents the hypothetical charge an atom would have if all bonds were completely ionic. While calculating oxidation states can be complex for certain molecules, understanding the key indicators allows for a simpler approach in many cases.
Here are some key indicators to look for:
1. Changes in Oxidation States:
This is the most fundamental indicator. If the oxidation state of at least one element changes during the reaction, it's a redox reaction. A change in oxidation state implies a gain or loss of electrons.
Example:
Fe²⁺ + Cu²⁺ → Fe³⁺ + Cu⁺
Iron's oxidation state increases from +2 to +3 (oxidation), while copper's oxidation state decreases from +2 to +1 (reduction). This is a redox reaction.
2. Presence of Oxidizing and Reducing Agents:
Oxidizing agents are substances that cause oxidation in other substances by accepting electrons themselves. They undergo reduction. Reducing agents are substances that cause reduction in other substances by donating electrons. They undergo oxidation. Identifying these agents often simplifies the redox reaction identification.
Example:
In the reaction 2Mg + O₂ → 2MgO, oxygen (O₂) is the oxidizing agent (it accepts electrons and is reduced), and magnesium (Mg) is the reducing agent (it donates electrons and is oxidized).
3. Specific Reactions Involving Oxygen, Hydrogen, or Halogens:
Reactions involving oxygen, hydrogen, or halogens often involve changes in oxidation states.
-
Reactions with Oxygen: Oxygen typically has an oxidation state of -2. If a substance reacts with oxygen and its oxidation state increases, it's been oxidized (and oxygen has been reduced).
-
Reactions with Hydrogen: Hydrogen usually has an oxidation state of +1. If a substance reacts with hydrogen and its oxidation state decreases, it's been reduced (and hydrogen has been oxidized).
-
Reactions with Halogens: Halogens (F, Cl, Br, I) usually have an oxidation state of -1. Reactions where halogens gain or lose electrons indicate a redox reaction.
4. Combination and Decomposition Reactions:
Many combination and decomposition reactions are redox reactions.
-
Combination reactions where elements combine to form compounds usually involve changes in oxidation states. For example, the formation of metal oxides from metals and oxygen is a redox reaction.
-
Decomposition reactions where a compound breaks down into its constituent elements often involve changes in oxidation states. For instance, the decomposition of hydrogen peroxide (H₂O₂) into water (H₂O) and oxygen (O₂) is a redox reaction.
Applying the Knowledge: Examples and Practice
Let's analyze several reactions and determine whether they are redox reactions:
1. 2H₂ + O₂ → 2H₂O
- Hydrogen: Oxidation state changes from 0 to +1 (oxidation)
- Oxygen: Oxidation state changes from 0 to -2 (reduction)
Conclusion: This is a redox reaction.
2. AgNO₃ + NaCl → AgCl + NaNO₃
- Silver (Ag): Oxidation state remains +1
- Nitrate (NO₃⁻): Oxidation state remains -1
- Sodium (Na): Oxidation state remains +1
- Chloride (Cl⁻): Oxidation state remains -1
Conclusion: This is not a redox reaction. It's a precipitation reaction (double displacement).
3. Zn + 2HCl → ZnCl₂ + H₂
- Zinc (Zn): Oxidation state changes from 0 to +2 (oxidation)
- Hydrogen (H): Oxidation state changes from +1 to 0 (reduction)
Conclusion: This is a redox reaction.
4. 2KClO₃ → 2KCl + 3O₂
- Chlorine (Cl): Oxidation state changes from +5 to -1 (reduction)
- Oxygen (O): Oxidation state changes from -2 to 0 (oxidation)
Conclusion: This is a redox reaction (decomposition reaction).
5. CaO + H₂O → Ca(OH)₂
- Calcium (Ca): Oxidation state remains +2
- Oxygen (O): Oxidation state remains -2
- Hydrogen (H): Oxidation state remains +1
Conclusion: This is not a redox reaction. It's an acid-base reaction.
Advanced Considerations: Disproportionation Reactions
A special type of redox reaction is a disproportionation reaction, also known as a self-redox reaction. In this type of reaction, a single element undergoes both oxidation and reduction simultaneously. The same element is both oxidized and reduced.
Example:
2Cu⁺ → Cu²⁺ + Cu
Copper(I) ions (Cu⁺) disproportionate into copper(II) ions (Cu²⁺) and copper metal (Cu). Copper is both oxidized (+1 to +2) and reduced (+1 to 0).
Troubleshooting and Common Mistakes
Even with clear guidelines, identifying redox reactions can sometimes be challenging. Here are some common mistakes to avoid:
-
Ignoring Spectator Ions: Focus on the species undergoing changes in oxidation state. Spectator ions (ions that remain unchanged throughout the reaction) don't affect whether a reaction is a redox reaction or not.
-
Incorrect Oxidation State Calculation: Mastering the rules for assigning oxidation states is essential. Practice calculating oxidation states for various compounds and ions to enhance your accuracy.
-
Assuming All Combination and Decomposition Reactions are Redox: While many are, not all combination and decomposition reactions are redox. Always check for changes in oxidation states.
Conclusion
Identifying redox reactions is a crucial skill in chemistry. By understanding the fundamental principles of oxidation and reduction, recognizing key indicators, and practicing with diverse examples, you can confidently determine whether a reaction involves the transfer of electrons. Remember to focus on changes in oxidation states, identify oxidizing and reducing agents, and be aware of special cases like disproportionation reactions. With practice and careful attention to detail, you'll master this essential aspect of chemical reactivity. Continuously reviewing examples and challenging yourself with more complex scenarios will solidify your understanding and enable you to apply this knowledge effectively in various chemical contexts.
Latest Posts
Latest Posts
-
Cuanto Es El 30 De 50
Mar 30, 2025
-
Spent Lead Acid Batteries Are Exempt From Hazardous Waste
Mar 30, 2025
-
How Many Centimeters Is 21 In
Mar 30, 2025
-
How Many Ounces Equals 2 Pounds
Mar 30, 2025
-
What Percent Of 50 Is 6
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Indicate Which Reactions Are Redox Reactions. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
