Is Baking Soda A Pure Substance
Kalali
Mar 10, 2025 · 5 min read
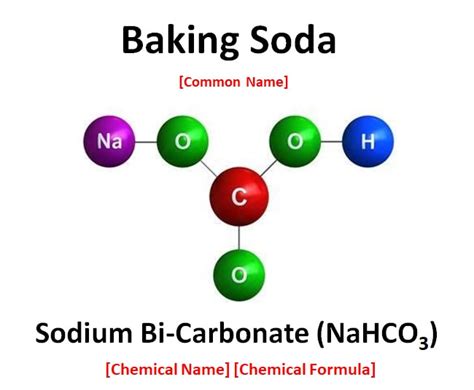
Table of Contents
Is Baking Soda a Pure Substance? A Deep Dive into Chemical Composition and Properties
Baking soda, a ubiquitous kitchen staple, is often assumed to be a simple substance. However, the answer to whether it's a pure substance is more nuanced than a simple yes or no. This article will explore the chemical composition of baking soda, delve into the definition of a pure substance, and ultimately answer the question definitively, while also exploring related concepts like mixtures and compounds.
Understanding Pure Substances
Before we classify baking soda, let's clarify what constitutes a pure substance. In chemistry, a pure substance is defined as a material that is composed of only one type of atom or molecule. This means that its composition is uniform and consistent throughout the sample. It cannot be separated into simpler substances by physical methods like filtration, distillation, or evaporation. Pure substances can be further categorized into elements and compounds.
-
Elements: Elements are substances made up of only one type of atom. Examples include oxygen (O), iron (Fe), and gold (Au). They are fundamental building blocks of matter and cannot be broken down into simpler substances by chemical means.
-
Compounds: Compounds are substances made up of two or more different types of atoms chemically bonded together in a fixed ratio. This bonding creates a new substance with unique properties different from its constituent elements. Water (H₂O), table salt (NaCl), and carbon dioxide (CO₂) are examples of compounds. They can only be separated into their constituent elements through chemical processes.
The Chemical Composition of Baking Soda
Baking soda, also known as sodium bicarbonate, has the chemical formula NaHCO₃. This formula tells us that each molecule of baking soda consists of one sodium atom (Na), one hydrogen atom (H), one carbon atom (C), and three oxygen atoms (O). These atoms are chemically bonded together, forming a specific molecular structure. This fixed ratio of atoms is a key characteristic of a compound.
Is Baking Soda a Mixture?
A mixture, unlike a pure substance, is a combination of two or more substances that are not chemically bonded. The components of a mixture retain their individual properties and can be separated by physical means. For example, a mixture of sand and salt can be separated by dissolving the salt in water and then filtering out the sand.
Baking soda, in its purest form, is not a mixture. It's a single compound with a consistent chemical composition. However, commercially produced baking soda may contain trace impurities depending on the manufacturing process. These impurities might include other sodium compounds or even residual water. But these impurities exist in such small quantities that they do not significantly alter the overall chemical identity of baking soda.
Baking Soda: A Compound, Not an Element
Since baking soda is composed of different types of atoms (sodium, hydrogen, carbon, and oxygen) chemically bonded together, it clearly isn't an element. Elements are composed of only one type of atom. Therefore, baking soda is classified as a compound.
Purity and Commercial Baking Soda
While pure sodium bicarbonate is a compound, the baking soda you purchase from the grocery store isn’t perfectly pure. Manufacturing processes can introduce trace amounts of impurities. This doesn't change the fundamental nature of baking soda as a compound, but it’s important to understand that "pure" in a commercial context doesn't always mean absolute chemical purity. The level of impurities is usually very low and doesn't affect the typical uses of baking soda in cooking or cleaning.
The Importance of Purity in Different Applications
The required purity of baking soda varies depending on its intended use. For everyday baking and cleaning, the level of impurities in commercially available baking soda is generally acceptable. However, for specific scientific experiments or industrial applications, a higher degree of purity might be necessary. In these cases, higher-grade baking soda with stricter purity specifications is available.
Distinguishing between Pure Substances and Mixtures: Practical Examples
To further solidify our understanding, let’s compare baking soda to some common mixtures:
-
Baking powder: Baking powder is a mixture of baking soda and other ingredients, typically an acid like cream of tartar and a starch. These components are not chemically bonded and can be separated by physical means.
-
Saltwater: Saltwater is a mixture of salt (NaCl, a compound) dissolved in water (H₂O, a compound). Salt and water retain their individual properties, and the mixture can be separated by evaporation.
-
Air: Air is a mixture of various gases, including nitrogen, oxygen, and carbon dioxide. These gases are not chemically bonded.
Conclusion: Baking Soda – A Compound, and Therefore, a Pure Substance
In essence, pure sodium bicarbonate (baking soda) is indeed a pure substance. It's a compound formed by the chemical combination of specific elements in a fixed ratio. While commercial baking soda might contain trace impurities, these are generally insignificant for common uses. The crucial distinction is that baking soda, at its core, is a single chemical entity, unlike mixtures that contain a combination of physically mixed substances. Understanding this distinction is essential for anyone interested in chemistry, baking, or scientific applications of this common household item.
Further Exploration: Related Chemical Concepts
This discussion naturally leads to further exploration of related chemical concepts. For instance, one might want to delve deeper into:
-
Stoichiometry: The study of the quantitative relationships between reactants and products in chemical reactions. Understanding stoichiometry allows for precise calculations of the amounts of reactants needed to produce a specific amount of product, relevant to baking and other applications.
-
Acid-base reactions: Baking soda is a base, and its reaction with acids (like those found in baking powder or acidic ingredients in recipes) is crucial to its leavening properties in baking. Studying acid-base chemistry helps explain this key feature.
-
Chemical bonding: Understanding ionic bonding, the type of bonding present in baking soda, gives insight into the strength and stability of the compound.
By understanding the chemical composition and properties of baking soda, we can appreciate its versatility and importance in diverse applications. This knowledge extends beyond the kitchen, providing a foundation for understanding more complex chemical concepts.
Latest Posts
Latest Posts
-
Food Chain In The Boreal Forest
Mar 10, 2025
-
10 Out Of 12 In Percentage
Mar 10, 2025
-
How Many Electrons Are In Chlorine
Mar 10, 2025
-
Least Common Multiple Of 14 And 21
Mar 10, 2025
-
How Many Hours Are In 90 Minutes
Mar 10, 2025
Related Post
Thank you for visiting our website which covers about Is Baking Soda A Pure Substance . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
