Is Boiling Water A Chemical Change
Kalali
Mar 20, 2025 · 5 min read
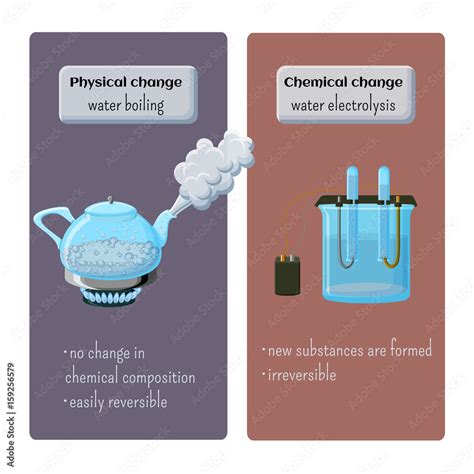
Table of Contents
Is Boiling Water a Chemical Change? A Deep Dive into Physical vs. Chemical Transformations
The question of whether boiling water represents a chemical or physical change is a common one, often sparking debate among students and enthusiasts of science alike. While seemingly simple, the answer requires a thorough understanding of the fundamental differences between physical and chemical changes, delving into the molecular behavior of water and the implications of its phase transitions. This comprehensive guide will explore this fascinating topic, providing a detailed explanation supported by scientific evidence.
Understanding Chemical and Physical Changes
Before tackling the central question, it's crucial to establish a clear definition of chemical and physical changes. This distinction forms the bedrock of understanding the transformation of water from liquid to gas.
Physical Changes: Altering Form, Not Composition
A physical change alters the form or appearance of a substance but doesn't change its chemical composition. Think of it as rearranging the furniture in a room – the individual pieces remain the same; only their arrangement has changed. Examples include:
- Changes of state: Melting ice, boiling water, freezing liquid, and sublimation (solid to gas).
- Shape changes: Cutting paper, bending a wire, crushing a can.
- Dissolution: Dissolving sugar in water (the sugar remains sugar).
Chemical Changes: Altering Composition
A chemical change, also known as a chemical reaction, involves the rearrangement of atoms and molecules, resulting in the formation of new substances with different chemical properties. This is akin to building a new piece of furniture from individual parts – the final product is entirely different from the components. Key indicators include:
- Gas production: Bubbles or fizzing.
- Color change: A significant and unexpected shift in color.
- Precipitate formation: The formation of a solid from a solution.
- Temperature change: A significant release or absorption of heat (exothermic or endothermic reaction).
- Irreversibility: The original substance cannot be easily recovered without further chemical reactions.
The Boiling of Water: A Detailed Examination
Now, let's apply this knowledge to the boiling of water. When water boils, it transitions from its liquid phase to its gaseous phase (steam). At a molecular level, this involves an increase in the kinetic energy of water molecules.
Molecular Behavior During Boiling
In liquid water, water molecules (H₂O) are relatively close together, held by relatively weak intermolecular forces (hydrogen bonds). These bonds are constantly breaking and reforming, allowing the molecules to move around each other. Heating the water increases the kinetic energy of these molecules.
As the temperature rises, the kinetic energy of the molecules overcomes the intermolecular forces holding them together. This allows the molecules to escape the liquid phase and enter the gaseous phase as steam. Crucially, the individual water molecules remain intact – they are still H₂O molecules.
No New Substances Formed
The boiling process does not create any new chemical substances. The steam produced is still composed of H₂O molecules, simply existing in a more dispersed state. There's no change in the chemical composition of the water; only a change in its physical state. No gases are produced other than the water vapor itself, and no color changes occur. While there is a significant temperature change, this is merely a reflection of the phase transition and not indicative of a chemical reaction. The process is easily reversible – by cooling the steam, it condenses back into liquid water.
Key Arguments Supporting Boiling Water as a Physical Change
Several lines of evidence strongly support the classification of boiling water as a physical change:
- Reversibility: Steam can easily be condensed back into liquid water, demonstrating the reversibility of the process. This is a hallmark characteristic of physical changes.
- No new substances are formed: The chemical composition of the water remains unchanged throughout the boiling process. The steam is still composed of H₂O molecules.
- Only a phase transition: The change involves only a change in the physical state of the water from liquid to gas, not a change in its chemical composition.
- No chemical indicators: None of the characteristic indicators of a chemical change (gas production, color change, precipitate formation) are present.
Addressing Potential Misconceptions
While the evidence overwhelmingly supports boiling water as a physical change, some misconceptions may arise:
- Decomposition: Some might argue that the separation of water into hydrogen and oxygen gases is a chemical change. However, this requires significant energy input (electrolysis) and does not occur naturally during boiling. Boiling simply changes the state of already existing water molecules.
- Energy change: The absorption of energy during boiling might seem to suggest a chemical reaction. However, energy is absorbed during many physical changes, like melting ice. The energy is used to overcome intermolecular forces, not to break chemical bonds.
- Steam vs. water: Steam and water are the same substance (H₂O), existing in different physical states.
Conclusion: Boiling Water Remains a Physical Transformation
In conclusion, boiling water unequivocally represents a physical change, not a chemical change. The process involves a change in the physical state of water from liquid to gas, driven by an increase in the kinetic energy of water molecules. Crucially, the chemical composition of the water remains unchanged. The molecules are merely rearranging themselves, moving further apart, and altering the phase they occupy. The reversibility of the process, the absence of any indicators of a chemical reaction, and the unchanging chemical composition of the water all firmly establish this transformation as a physical phenomenon. This understanding is essential for grasping fundamental concepts in chemistry and physics, emphasizing the importance of distinguishing between changes in substance and changes in state.
Latest Posts
Latest Posts
-
Cuanto Es 100 Centimetros En Pulgadas
Mar 21, 2025
-
Adaptations For Animals In The Grasslands
Mar 21, 2025
-
138 Inches Is How Many Feet
Mar 21, 2025
-
How Will Grazing Animals Help Plants To Become Established
Mar 21, 2025
-
Is Aluminum Foil A Pure Substance
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Is Boiling Water A Chemical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
