Is Gold An Element Compound Or Mixture
Kalali
Mar 28, 2025 · 5 min read
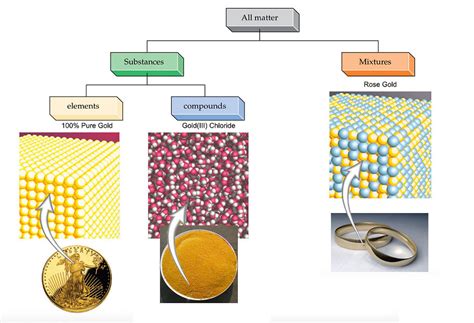
Table of Contents
Is Gold an Element, Compound, or Mixture? A Deep Dive into the Nature of Gold
Gold. The word itself conjures images of shimmering treasures, ancient empires, and enduring value. But beyond its cultural and economic significance, what is gold at its most fundamental level? Is it an element, a compound, or a mixture? The answer, as we'll explore in detail, is surprisingly straightforward, yet understanding the underlying principles reveals a deeper appreciation for this fascinating metal.
Understanding the Basic Classifications of Matter
Before diving into the specifics of gold, let's establish a clear understanding of the three basic classifications of matter: elements, compounds, and mixtures. This foundation will help us definitively categorize gold and appreciate its unique properties.
Elements: The Building Blocks of Matter
Elements are pure substances that cannot be broken down into simpler substances by chemical means. They are fundamental building blocks of all matter, represented by their unique symbols on the periodic table. Each element is characterized by a specific number of protons in its atomic nucleus, called its atomic number. Examples include oxygen (O), hydrogen (H), and, crucially for our discussion, gold (Au).
Compounds: Elements Bound Together
Compounds are formed when two or more elements chemically combine in fixed proportions. This combination involves the sharing or transfer of electrons, creating strong chemical bonds. The properties of a compound are distinctly different from the properties of its constituent elements. For example, water (H₂O) is a compound formed from hydrogen and oxygen; it's a liquid at room temperature, unlike its gaseous components.
Mixtures: A Blend of Substances
Mixtures are combinations of two or more substances that are not chemically bonded. They can be separated into their components by physical means, such as filtration or distillation. Mixtures retain the properties of their individual components. Examples include air (a mixture of gases) and saltwater (a mixture of salt and water).
Gold: A Definitive Element
Now, armed with these definitions, we can confidently state that gold is an element. It's a pure substance with the atomic number 79, represented by the symbol Au (from the Latin word aurum). Gold cannot be broken down into simpler substances through chemical reactions. Its unique properties, such as its characteristic yellow color, malleability, ductility, and resistance to corrosion, stem directly from its atomic structure and electron configuration.
The Atomic Structure of Gold
Gold's atomic structure is crucial to understanding its properties. It has 79 protons and typically 118 neutrons in its nucleus, giving it an atomic mass of approximately 197 atomic mass units (amu). The arrangement of its electrons in shells and subshells dictates its chemical behavior and physical properties. The outermost electrons are relatively loosely held, contributing to gold's malleability and ductility – its ability to be easily hammered into thin sheets and drawn into wires.
Gold's Resistance to Corrosion: A Key Feature
Gold's resistance to corrosion is another hallmark of its elemental nature. Unlike many metals that readily react with oxygen or other substances in the environment, gold is exceptionally unreactive. This inertness is due to its electronic configuration; it has a full outer electron shell, making it stable and resistant to oxidation. This resistance is a key reason why gold has been valued for millennia, retaining its luster and beauty for extended periods.
Differentiating Gold from its Alloys and Mixtures
While pure gold is an element, it's rarely found in its pure form in nature. Often, gold is alloyed with other metals to enhance its durability and other properties. These alloys are mixtures, not compounds.
Alloys: Mixtures, Not Compounds
Gold alloys are mixtures of gold with other metals, such as copper, silver, or nickel. These additions alter the gold's properties, such as its hardness and color. For example, 14-karat gold is a mixture containing approximately 58.3% gold, and the remaining percentage consists of other metals, primarily copper and silver. These alloys are still considered mixtures because the component metals are not chemically bonded. The metals are physically combined, and they can be separated through techniques like electrolysis.
Gold in its Natural State: Often a Mixture
Even in its natural state, gold is rarely found as pure, elemental gold. Gold nuggets and deposits often contain impurities—other elements or compounds mixed in with the gold. These impurities can include quartz, other minerals, or even traces of other metals. These naturally occurring formations are considered mixtures because the gold isn’t chemically bonded to the other components. These mixtures can be separated through various techniques like panning, smelting, or chemical purification processes.
Why Understanding the Classification Matters
Understanding that gold is an element, not a compound or simply a mixture, clarifies its fundamental nature and behavior. This knowledge is crucial in various fields:
-
Jewelry and Metalworking: The properties of gold, its malleability, and ductility are directly related to its elemental nature. This is essential for shaping and crafting gold into various forms. The addition of other metals to create alloys further demonstrates the understanding of its properties as an element.
-
Investment and Finance: Gold's value as a precious metal is linked to its rarity, resistance to corrosion, and inherent properties as a chemically stable element. Understanding its chemical inertness ensures investors know it will maintain its value without degrading over time.
-
Scientific Research: In scientific studies, the understanding of gold's elemental nature is fundamental to its use in various applications, such as in electronics, medicine, and catalysis. The unique atomic structure and electron configuration influence its specific applications.
-
Archaeology and History: The enduring nature of gold, its resistance to degradation and alteration, plays a key role in preserving artifacts and historical objects made of gold across centuries. Understanding its fundamental stability enables us to better interpret the history it preserves.
Conclusion: Gold's Enduring Elemental Nature
In conclusion, gold is definitively an element. Its unique properties, from its brilliant yellow color to its resistance to corrosion, stem directly from its atomic structure and elemental nature. While it’s often found mixed with other substances in nature or alloyed with other metals to enhance its practical applications, the fundamental building block remains the pure element gold (Au). This elemental status explains its enduring value, both culturally and economically, and its significant applications across many fields of science and technology. Understanding this essential truth provides a richer appreciation for this precious and fascinating metal.
Latest Posts
Latest Posts
-
Water Is Always A Product In What Type Of Reaction
Mar 31, 2025
-
How Much Is A Half A Pint In Cups
Mar 31, 2025
-
Convert 18 Degrees C To Fahrenheit
Mar 31, 2025
-
What Is 33 C In Fahrenheit
Mar 31, 2025
-
24 Is What Percent Of 30
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Is Gold An Element Compound Or Mixture . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
