Is Reactivity A Physical Or Chemical Property
Kalali
Mar 24, 2025 · 5 min read
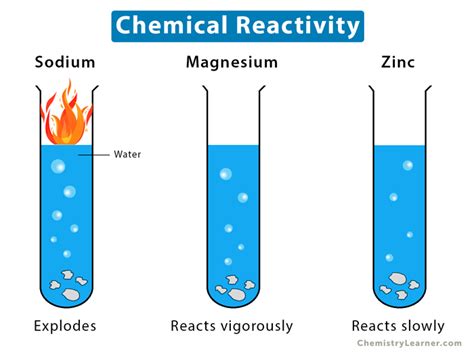
Table of Contents
Is Reactivity a Physical or Chemical Property?
Reactivity, a fundamental concept in chemistry, often leaves students questioning its classification. Is it a physical property, like color or density, or a chemical property, like flammability or acidity? The answer, as with many things in science, is nuanced. While reactivity is intrinsically linked to chemical changes, its manifestation and measurement can involve both physical and chemical observations. This article delves into the complexities of reactivity, exploring its nature, measurement, and the reasons why assigning it solely to one category proves challenging.
Understanding Reactivity: A Definition
Before classifying reactivity, let's precisely define it. Reactivity refers to the tendency of a substance to undergo a chemical change. This chemical change can manifest in various ways, including reactions with other substances, decomposition, or even spontaneous changes within the substance itself. A highly reactive substance will readily undergo chemical reactions, while a less reactive substance will be slower or require specific conditions to react. This inherent tendency is what makes reactivity a key characteristic of matter, informing its applications and safety considerations.
Factors Influencing Reactivity
Numerous factors influence a substance's reactivity. These include:
-
Electron Configuration: The arrangement of electrons in an atom's outermost shell (valence electrons) is crucial. Atoms with incomplete valence shells strive to achieve stability by gaining, losing, or sharing electrons, leading to chemical reactions. Elements with highly reactive valence electron configurations, like alkali metals (Group 1) and halogens (Group 17), are prime examples.
-
Electronegativity: This property describes an atom's ability to attract electrons towards itself in a chemical bond. High electronegativity differences between reacting atoms lead to more vigorous reactions.
-
Ionization Energy: The energy required to remove an electron from an atom influences reactivity. Low ionization energy indicates easier electron removal, increasing reactivity.
-
Bond Strength: The strength of bonds within a molecule dictates its stability and reactivity. Weaker bonds are more easily broken, leading to higher reactivity.
-
Surface Area: For solids, the surface area exposed to other reactants plays a significant role. Increased surface area means more contact points for reactions, thus increasing reactivity. This is why finely powdered substances are often more reactive than their bulk counterparts.
-
Temperature and Pressure: These factors affect the kinetic energy of particles. Higher temperatures and pressures generally lead to increased collision frequency and energy, boosting reaction rates and potentially influencing reactivity.
-
Presence of Catalysts: Catalysts accelerate chemical reactions without being consumed themselves. They provide alternative reaction pathways with lower activation energy, thus influencing the overall reactivity of a system.
The Case for Reactivity as a Chemical Property
The primary argument for classifying reactivity as a chemical property stems from its direct relationship with chemical changes. Reactivity is, by definition, the propensity for a substance to undergo chemical transformations, forming new substances with different properties. These transformations involve the breaking and formation of chemical bonds, resulting in changes to the molecular structure and composition.
For example, the reactivity of sodium (Na) is evident in its vigorous reaction with water, producing sodium hydroxide (NaOH) and hydrogen gas (H₂). This is a clear chemical change, characterized by a new substance formation and energy release (exothermic reaction). Similarly, the inertness of noble gases like helium (He) is a consequence of their stable electron configurations, demonstrating their low reactivity as a chemical property.
Observing reactivity often requires inducing a chemical change. This necessitates interaction with another substance or altering conditions to trigger a reaction, hence directly linking it to chemical behavior. This contrasts with physical properties that can be observed without altering the substance's chemical composition.
The Case for Reactivity's Physical Manifestations
Although inherently a chemical property, the manifestation of reactivity often involves observable physical changes. The evidence of a chemical reaction frequently includes physical phenomena like:
-
Color Change: A change in color is a common visual indicator of a chemical reaction, showcasing reactivity indirectly.
-
Gas Evolution: The production of gas bubbles signifies a chemical reaction, demonstrating the substance's reactivity.
-
Precipitation: Formation of a solid precipitate indicates a chemical reaction has occurred, reflecting the reactivity of the involved substances.
-
Temperature Change: Exothermic reactions (releasing heat) or endothermic reactions (absorbing heat) provide physical evidence of reactivity via temperature changes.
-
Light Emission: Some reactions are accompanied by light emission (chemiluminescence), further showing physical evidence of a chemical process and, thereby, reactivity.
These physical observations are consequences of the underlying chemical changes driven by reactivity. While not directly defining reactivity itself, they serve as crucial indicators and methods for measuring and quantifying it. Therefore, it's accurate to state that while reactivity is fundamentally a chemical property, its measurement and observation often involve physical properties.
Quantifying Reactivity: A Blend of Chemical and Physical Methods
Measuring and quantifying reactivity involves experimental techniques incorporating both chemical and physical principles. Several approaches exist, including:
-
Reaction Rate Measurements: Determining the rate at which a reaction proceeds provides quantitative information about reactivity. This often involves measuring physical changes over time, such as concentration changes (using techniques like spectrophotometry) or gas volume evolution.
-
Activation Energy Determination: Measuring the activation energy—the minimum energy required for a reaction to occur—provides insight into a substance's reactivity. Techniques like differential scanning calorimetry (DSC) are often employed.
-
Electrochemical Methods: For redox reactions, electrochemical techniques (like potentiometry) can be used to measure the potential difference between electrodes and determine the reactivity of substances.
-
Stability Studies: Monitoring the degradation or decomposition of a substance over time under various conditions provides valuable data on its inherent stability and, hence, its reactivity.
All these methods fundamentally assess chemical changes but rely heavily on the measurement of physical parameters.
Conclusion: The Dual Nature of Reactivity
In conclusion, reactivity is best understood as a chemical property because it directly relates to a substance's inherent tendency to undergo chemical changes. However, its manifestation and measurement frequently involve the observation and quantification of physical properties. The dynamic interplay between chemical and physical aspects makes classifying reactivity solely as either a chemical or a physical property overly simplistic. It’s more accurate to view reactivity as a chemical property whose characterization involves a blend of chemical and physical techniques. This understanding is crucial for predicting chemical behavior, designing chemical processes, and ensuring the safe handling of reactive materials. The seemingly simple concept of reactivity reveals a sophisticated interaction between chemical transformations and their observable physical manifestations. Therefore, a comprehensive understanding requires a holistic approach that embraces both perspectives.
Latest Posts
Latest Posts
-
How Many Kilometers Is 3000 Miles
Mar 28, 2025
-
How Many Cups Of Water Is 5 Quarts
Mar 28, 2025
-
220 Celsius Is What In Fahrenheit
Mar 28, 2025
-
How Many Pints Is A Liter
Mar 28, 2025
-
What Is The Solution Of This Linear System
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Is Reactivity A Physical Or Chemical Property . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
