Is Snow Solid Liquid Or Gas
Kalali
Mar 18, 2025 · 6 min read
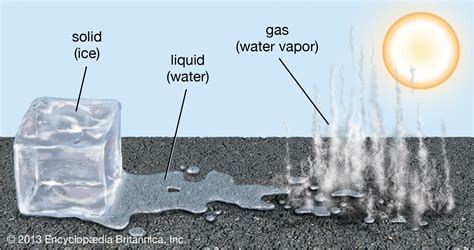
Table of Contents
Is Snow Solid, Liquid, or Gas? Understanding the Phases of Water
Snow, that beautiful, delicate, and often disruptive blanket that covers the world in winter, presents a deceptively simple question: what is it? Is snow a solid, a liquid, or a gas? The answer, like many things in science, is more nuanced than a simple one-word response. While seemingly straightforward, understanding the nature of snow requires delving into the fascinating world of water's phases and the intricate processes that govern its transformation.
The Three Main States of Matter: Solid, Liquid, and Gas
Before diving into the specifics of snow, let's establish a fundamental understanding of the three primary states of matter:
-
Solid: In a solid state, molecules are tightly packed together in a fixed arrangement, held in place by strong intermolecular forces. This structure gives solids their characteristic rigidity and definite shape and volume. Think of an ice cube – its shape remains consistent unless an external force changes it.
-
Liquid: Liquids possess a definite volume but lack a definite shape. Their molecules are still relatively close together but have more freedom of movement compared to solids. This allows liquids to flow and conform to the shape of their container. Water in a glass is a perfect example; it takes the shape of the glass but maintains a consistent volume.
-
Gas: Gases have neither a definite shape nor a definite volume. Their molecules are widely dispersed and move independently with high kinetic energy, leading to their ability to expand to fill any available space. Think of steam; it expands to fill the entire room.
Snow: A Closer Look at its Composition
Now, let's focus our attention on snow. Snow is essentially a collection of ice crystals. Ice, of course, is water in its solid state. Therefore, the fundamental building block of snow is a solid. Each snowflake begins as a tiny ice crystal that forms around a microscopic particle in the atmosphere, such as dust or pollen. As more water vapor freezes onto this nucleus, it forms intricate and often unique crystalline structures.
However, the story doesn't end there. The seemingly simple statement "snow is solid" overlooks several crucial aspects:
The Role of Water Vapor
Snow's formation is inextricably linked to water vapor, which is water in its gaseous state. The transition from gaseous water vapor to solid ice crystals is a process called deposition. This is different from condensation (gas to liquid) and then freezing (liquid to solid). In deposition, the water vapor directly transitions from a gas to a solid, bypassing the liquid phase. This process happens in the atmosphere as temperatures drop below freezing.
The Presence of Liquid Water within Snowpack
While snow itself is primarily composed of solid ice crystals, a snowpack – a significant accumulation of snow – can contain a surprising amount of liquid water. This liquid water exists in various forms:
-
Liquid water films: Thin layers of liquid water can coat the surfaces of ice crystals, particularly in warmer conditions or when the snowpack is undergoing melting.
-
Liquid water within the snowpack: As the snowpack compresses under its own weight, particularly at lower depths, the spaces between the ice crystals can fill with liquid water. This is known as meltwater and significantly impacts the snowpack's stability and hydrological properties.
-
Water vapor within the snowpack: Even in extremely cold conditions, a certain amount of water vapor can exist within the spaces of the snowpack. This water vapor can participate in sublimation and deposition processes within the snowpack itself.
Snow's Diverse Properties and Behaviors
The interplay between the solid ice crystals, liquid water, and water vapor within a snowpack results in a variety of properties and behaviors that complicate a simple classification:
-
Density: Snow's density varies greatly depending on factors such as temperature, crystal shape, and the amount of liquid water present. Freshly fallen snow can be very fluffy and have a low density, while older snowpacks that have undergone compaction can become much denser.
-
Metamorphism: Snow undergoes continuous change or metamorphism after its initial formation. This includes processes such as sintering (the bonding of ice crystals), recrystallization (changes in crystal shape and size), and sublimation (the direct transition from solid ice to gaseous water vapor). These processes alter the snowpack's structure and properties over time.
-
Mechanical Properties: The mechanical properties of snow, such as its strength and ability to support weight, are heavily influenced by its density, temperature, and the amount of liquid water present. These properties are critical in various applications, including avalanche forecasting and winter sports.
The Importance of Context
Therefore, classifying snow simply as solid, liquid, or gas is an oversimplification. While the fundamental building blocks of snow are solid ice crystals, the presence of liquid water and water vapor within the snowpack, alongside the ongoing processes of metamorphism, add significant complexity. The most accurate description depends heavily on the context:
- A single snowflake: Primarily solid ice.
- A freshly fallen snowpack: Predominantly solid with minor amounts of liquid water potentially present on crystal surfaces.
- An older, compacted snowpack: A mixture of solid ice, liquid water, and possibly some water vapor.
Beyond the Three States: A More Comprehensive View
The discussion about snow's state highlights a limitation of simply classifying matter as solid, liquid, or gas. While these three states are essential for a basic understanding, many materials exhibit more complex behavior that doesn't fit neatly into these categories. Snow provides a good example of this complexity. Consider these additional aspects:
-
Amorphous Solids: While most ice is crystalline, some forms of ice can exist in an amorphous state, lacking the long-range order characteristic of crystalline solids. These amorphous forms may have properties that differ from crystalline ice.
-
Colloidal Systems: Snow can be considered a colloidal system, where ice crystals are dispersed within a medium containing liquid water and water vapor. Understanding the behavior of these complex systems is crucial to predicting snowpack stability and other snow-related phenomena.
-
Phase Transitions: The continuous transitions between solid, liquid, and gaseous phases within a snowpack make a simple classification challenging. The snowpack is constantly evolving, shifting between these states in response to changes in temperature, pressure, and other environmental factors.
Conclusion: The Complexity of Snow
Ultimately, the question, "Is snow solid, liquid, or gas?" doesn't have a straightforward answer. While the primary constituent of snow is solid ice, the presence of liquid water and water vapor, along with the complex processes of metamorphism, makes a simple categorization inadequate. Understanding snow requires acknowledging its multifaceted nature and the continuous interplay between its different phases. This complex interaction shapes its diverse properties and behaviors, influencing everything from weather patterns to avalanche risk, showcasing the beauty and wonder of this seemingly simple winter phenomenon. Therefore, understanding snow requires appreciating its dynamic and multifaceted nature as a complex material, rather than simply assigning it to a single state of matter.
Latest Posts
Latest Posts
-
High Frequency Wave Vs Low Frequency Wave
Mar 19, 2025
-
Temperate Deciduous Forest Oak Tree Adaptations
Mar 19, 2025
-
What Animals Can See Human Bioluminescence
Mar 19, 2025
-
Is Sour Taste A Physical Property
Mar 19, 2025
-
How Many Kilos Are 20 Pounds
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Is Snow Solid Liquid Or Gas . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
