Is Solubility A Physical Or Chemical Change
Kalali
Mar 22, 2025 · 6 min read
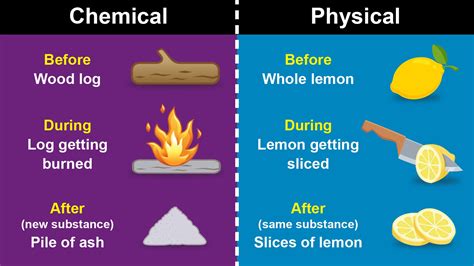
Table of Contents
Is Solubility a Physical or Chemical Change? A Deep Dive
The question of whether solubility represents a physical or chemical change is a surprisingly nuanced one, often sparking debate among students and scientists alike. While at first glance it might seem straightforward, a deeper understanding reveals a more complex picture involving both physical and chemical processes depending on the specific solute and solvent involved. This article will explore the intricacies of solubility, differentiating between the physical processes of dissolution and the chemical changes that can sometimes accompany it.
Understanding Solubility: The Basics
Solubility refers to the ability of a substance, the solute, to dissolve in a solvent to form a homogeneous solution. The solvent is typically a liquid, although it can also be a solid or a gas. The extent to which a solute dissolves in a solvent is expressed as its solubility, often quantified as the maximum amount of solute that can dissolve in a given amount of solvent at a specific temperature and pressure.
For example, sugar readily dissolves in water, meaning it has high solubility in water. Conversely, sand has very low solubility in water. This difference arises from the intermolecular forces between the solute and solvent molecules.
The Physical Aspects of Dissolution
The process of dissolving, where a solute disperses uniformly throughout a solvent, is fundamentally a physical change. This is because the chemical identity of both the solute and solvent remains unchanged. No new chemical bonds are formed or broken during the dissolution process.
Here's a breakdown of the physical processes involved:
1. Breaking Intermolecular Forces in the Solute:
Before a solute can dissolve, the attractive forces holding its molecules or ions together must be overcome. For example, in a solid crystalline structure like salt (NaCl), strong ionic bonds hold the sodium (Na⁺) and chloride (Cl⁻) ions together. To dissolve, these bonds are not broken, but the intermolecular forces holding the crystal lattice are weakened and eventually overcome by the solvent's interaction with the solute particles.
2. Breaking Intermolecular Forces in the Solvent:
Similarly, the solvent molecules must overcome their own intermolecular attractions to make room for the solute particles. Water molecules, for instance, are held together by hydrogen bonds. Dissolution requires these bonds to be temporarily disrupted to accommodate the incoming solute molecules or ions.
3. Formation of Solute-Solvent Interactions:
The key to successful dissolution lies in the formation of new attractions between the solute and solvent molecules or ions. These attractive forces are usually weaker than the original bonds within the solute and solvent, but strong enough to overcome the energy required for separation. These new attractive forces are responsible for the stabilization of the dissolved solute particles within the solvent. This interaction is often referred to as solvation, and when the solvent is water, it's specifically called hydration.
These three steps – breaking solute-solute interactions, breaking solvent-solvent interactions, and forming solute-solvent interactions – are all physical processes. No new chemical substances are created; only the state of matter and spatial arrangement of molecules are altered. The solute and solvent particles can, in principle, be recovered in their original forms through physical methods like evaporation or distillation.
When Solubility Becomes a Chemical Change
While dissolution itself is primarily a physical change, certain solutes may undergo chemical reactions upon dissolving. In such cases, solubility becomes intertwined with chemical changes, blurring the lines between the two.
1. Ionization and Dissociation:
Ionic compounds like salts dissociate into their constituent ions when dissolved in polar solvents like water. For example, NaCl dissolves in water to form Na⁺ and Cl⁻ ions. While this involves the breaking of ionic bonds within the crystal lattice, the ions themselves remain chemically unchanged; it's the separation and dispersal that constitutes the physical change.
However, the interaction of these ions with water molecules can be considered a chemical interaction, as the water molecules become oriented around the ions (hydration), leading to changes in the properties of both the ions and the water. This is a borderline case where the chemical interactions are subtle compared to a full-blown chemical reaction.
2. Chemical Reactions with the Solvent:
Some solutes react chemically with the solvent, forming new compounds. This is a clear indication of a chemical change, where the chemical identity of the original solute is altered.
For example, dissolving sodium metal (Na) in water leads to a vigorous reaction producing sodium hydroxide (NaOH) and hydrogen gas (H₂):
2Na(s) + 2H₂O(l) → 2NaOH(aq) + H₂(g)
In this instance, the sodium metal undergoes a chemical reaction with water, resulting in the formation of entirely new substances. The solubility of sodium in water is therefore inextricably linked to a chemical process.
3. Complex Formation:
Certain metal ions can form complex ions when dissolved in specific solvents. This involves the formation of coordinate covalent bonds between the metal ion and ligand molecules (usually from the solvent or added substances). This complexation changes the chemical properties of the metal ion, making it distinct from its original form. While the original metal ion's identity remains, its chemical behaviour is altered significantly. This process is a chemical change intertwined with the physical act of dissolving.
4. Hydrolysis:
Some salts undergo hydrolysis when dissolved in water, leading to a change in the pH of the solution. This involves a chemical reaction between the ions of the salt and water molecules, forming hydronium (H₃O⁺) or hydroxide (OH⁻) ions. The pH change is a clear indicator that a chemical reaction is accompanying the dissolution. For example, the dissolution of sodium acetate (NaCH₃COO) in water leads to the formation of some acetic acid and hydroxide ions, resulting in a slightly basic solution.
Distinguishing Physical from Chemical Changes in Solubility
The key to determining whether solubility involves a chemical change lies in examining the products formed after dissolution.
-
If the solute particles remain chemically unchanged after dissolving, even if they are dissociated or hydrated, the process is predominantly physical. The original solute can, in principle, be recovered by removing the solvent.
-
If new chemical substances are formed as a result of dissolution, the process involves a chemical change. The original solute cannot be recovered in its original form.
Conclusion: A Spectrum of Changes
The nature of solubility isn't a simple binary; it exists on a spectrum. In many cases, dissolution is a primarily physical process, involving the separation and dispersal of solute particles within the solvent. However, certain solutes can undergo chemical reactions during dissolution, complicating the picture. Understanding the specific interactions between the solute and solvent is crucial to accurately characterizing the nature of the change. Considering ionization, complex formation, hydrolysis, and chemical reactions with the solvent allows for a more comprehensive and accurate assessment of whether solubility is primarily a physical or chemical change in a given system. Careful observation of the products and changes in chemical properties helps to determine if a chemical reaction is taking place alongside the physical process of dissolution.
Latest Posts
Latest Posts
-
Unit 2 Cell Test Biology Practice
Mar 23, 2025
-
How Does Current Affect Biodiversity In Rivers And Streams
Mar 23, 2025
-
How Many Millimeters Are In 1 In
Mar 23, 2025
-
How Many Ml Is In 3oz
Mar 23, 2025
-
The Number Of Orbitals For The D Sublevel Is
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about Is Solubility A Physical Or Chemical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
