The Number Of Orbitals For The D Sublevel Is
Kalali
Mar 23, 2025 · 6 min read
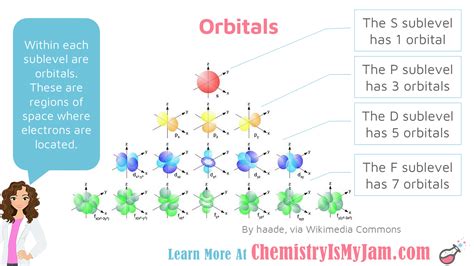
Table of Contents
The Number of Orbitals for the d Sublevel Is: A Deep Dive into Atomic Structure
The question, "the number of orbitals for the d sublevel is," seems simple enough. The answer, however, opens the door to a fascinating exploration of atomic structure, quantum numbers, and the implications of electron configuration. This article will not only answer the titular question definitively but also delve into the underlying principles that govern the arrangement of electrons within atoms, making it relevant for students of chemistry and physics alike.
Understanding Atomic Orbitals
Before we address the specific case of the d sublevel, it's crucial to understand the basic concept of atomic orbitals. In the quantum mechanical model of the atom, electrons don't orbit the nucleus in neatly defined paths like planets around a star. Instead, they exist in regions of space called atomic orbitals, which describe the probability of finding an electron at a particular location. These orbitals are characterized by a set of quantum numbers.
The Four Quantum Numbers
Four quantum numbers are necessary to completely describe the state of an electron in an atom:
-
Principal Quantum Number (n): This number dictates the energy level of the electron and the size of the orbital. It's a positive integer (n = 1, 2, 3,...). Higher values of n correspond to higher energy levels and larger orbitals.
-
Azimuthal Quantum Number (l): This number describes the shape of the orbital and its angular momentum. It can take integer values from 0 to n - 1. Each value of l corresponds to a subshell:
- l = 0: s subshell (spherical shape)
- l = 1: p subshell (dumbbell shape)
- l = 2: d subshell (more complex shapes)
- l = 3: f subshell (even more complex shapes)
-
Magnetic Quantum Number (ml): This number specifies the orientation of the orbital in space. It can take integer values from -l to +l, including 0. This means that for each subshell, there are (2l + 1) orbitals with different orientations.
-
Spin Quantum Number (ms): This number describes the intrinsic angular momentum (spin) of the electron. It can have only two values: +1/2 (spin up) or -1/2 (spin down). This is often represented by arrows ↑ and ↓.
Delving into the d Sublevel
Now, let's focus on the d sublevel. As mentioned earlier, the d sublevel corresponds to an azimuthal quantum number of l = 2. Using the formula (2l + 1), we can calculate the number of orbitals in the d subshell:
(2 * 2 + 1) = 5
Therefore, the number of orbitals for the d sublevel is 5.
These five d orbitals have distinct shapes and orientations in space, often designated as d<sub>xy</sub>, d<sub>xz</sub>, d<sub>yz</sub>, d<sub>x²-y²</sub>, and d<sub>z²</sub>. The subscripts indicate the orientation of the orbitals relative to the x, y, and z axes. Understanding these shapes is crucial in comprehending chemical bonding and molecular geometry.
Visualizing the d Orbitals
While precise graphical representations can be complex, it's helpful to visualize the general shapes:
-
d<sub>xy</sub>, d<sub>xz</sub>, d<sub>yz</sub>: These orbitals have four lobes arranged between the axes.
-
d<sub>x²-y²</sub>: This orbital has four lobes arranged along the x and y axes.
-
d<sub>z²</sub>: This orbital has two lobes along the z-axis and a ring in the xy-plane.
Implications of the Five d Orbitals
The existence of five d orbitals has significant implications in various areas of chemistry and physics:
-
Transition Metal Chemistry: Transition metals are characterized by partially filled d orbitals. The varying number of electrons in these orbitals leads to a rich and diverse range of oxidation states, complex ion formation, and catalytic activity. The unique shapes and orientations of d orbitals also influence the geometry of complexes.
-
Spectroscopy: The energy differences between the d orbitals in transition metal complexes play a crucial role in determining the colors of these compounds. This is due to the absorption of light in the visible region of the electromagnetic spectrum, promoting electrons from lower-energy d orbitals to higher-energy d orbitals. This phenomenon is explored in detail in spectroscopic techniques like UV-Vis spectroscopy.
-
Magnetism: The presence of unpaired electrons in d orbitals leads to paramagnetism in transition metal compounds. The number of unpaired electrons and their interactions determine the magnetic properties of these materials.
-
Catalysis: The ability of transition metals to easily change their oxidation states and the availability of d orbitals for bonding make them excellent catalysts in many chemical reactions.
-
Solid State Physics: The d orbitals contribute significantly to the electronic and magnetic properties of many solids, especially transition metal oxides and other materials with important technological applications.
Beyond the Basics: Filling the d Orbitals
Understanding the number of orbitals is only half the battle. The actual filling of these orbitals with electrons follows Hund's rule and the Aufbau principle.
-
Hund's Rule: Electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. This minimizes electron-electron repulsion and leads to a more stable configuration.
-
Aufbau Principle: Electrons fill orbitals in order of increasing energy. The filling order for the d orbitals is not always straightforward and can vary slightly depending on the specific atom and its surrounding environment.
This electron configuration dictates the chemical and physical properties of the atom. For instance, the electronic configuration of chromium (Cr) is unexpectedly [Ar] 3d⁵ 4s¹, rather than the expected [Ar] 3d⁴ 4s², due to the extra stability gained by having half-filled d and s subshells. These exceptions highlight the complexity and fascinating nuances of atomic structure.
Advanced Concepts and Further Exploration
The discussion above provides a solid foundation for understanding the number of d orbitals and their significance. However, several advanced concepts warrant further exploration for a more complete picture:
-
Ligand Field Theory: This theory extends the basic principles of crystal field theory by incorporating the covalent aspects of metal-ligand interactions. It provides a more nuanced understanding of the electronic structure of transition metal complexes.
-
Molecular Orbital Theory: This theory explains bonding in molecules by considering the combination of atomic orbitals to form molecular orbitals. It’s particularly important for understanding bonding in transition metal complexes.
-
Relativistic Effects: For heavier elements, relativistic effects become increasingly significant, impacting the energies and shapes of atomic orbitals, including those in the d sublevel. These effects are crucial for accurately predicting the properties of these elements.
Conclusion
In conclusion, the number of orbitals for the d sublevel is 5. This seemingly simple answer opens a vast landscape of atomic structure and its far-reaching implications across chemistry, physics, and materials science. Understanding the quantum numbers, orbital shapes, filling order, and the influence on chemical and physical properties is essential for anyone seeking a deeper comprehension of the atomic world. The exploration of d orbitals offers a gateway to numerous advanced concepts, revealing the intricate beauty and complexity of matter at its fundamental level. Further study into these related topics will undoubtedly enrich your understanding of this fascinating area of science.
Latest Posts
Latest Posts
-
How Many Inches Is 173 Cm
Mar 25, 2025
-
How Much Is 64 Oz In Liters
Mar 25, 2025
-
A Velocity Time Graph Shows How Velocity Changes Over
Mar 25, 2025
-
What Is 77 Inches In Feet
Mar 25, 2025
-
How Much Is 4 Quarts Of Water In Cups
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about The Number Of Orbitals For The D Sublevel Is . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
