Naoh + Hcl Net Ionic Equation
Kalali
Mar 27, 2025 · 5 min read
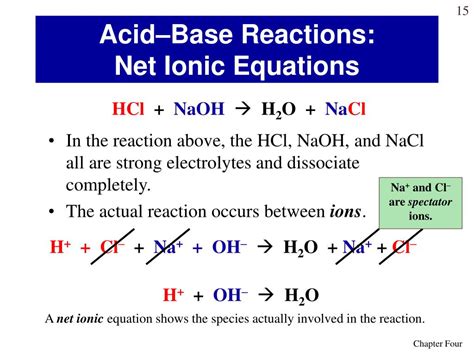
Table of Contents
NaOH + HCl: A Deep Dive into the Net Ionic Equation and its Significance
The seemingly simple reaction between sodium hydroxide (NaOH) and hydrochloric acid (HCl) offers a rich learning opportunity in chemistry, particularly concerning net ionic equations and the concepts of strong electrolytes, spectator ions, and acid-base neutralization. This comprehensive guide will explore this reaction in detail, explaining the underlying principles and its implications.
Understanding the Reactants: NaOH and HCl
Before diving into the net ionic equation, let's establish a strong foundation by understanding the individual reactants:
Sodium Hydroxide (NaOH)
Sodium hydroxide, commonly known as lye or caustic soda, is a strong base. This means it completely dissociates in aqueous solution, releasing sodium ions (Na⁺) and hydroxide ions (OH⁻):
NaOH(aq) → Na⁺(aq) + OH⁻(aq)
The presence of hydroxide ions is what gives NaOH its basic properties. It's crucial to remember that the complete dissociation is key to understanding its behavior in reactions.
Hydrochloric Acid (HCl)
Hydrochloric acid is a strong acid. Similar to NaOH, it completely dissociates in water, producing hydrogen ions (H⁺) and chloride ions (Cl⁻):
HCl(aq) → H⁺(aq) + Cl⁻(aq)
The abundance of hydrogen ions (often represented as hydronium ions, H₃O⁺, in more accurate representations) is what confers the acidic properties to HCl.
The Complete Ionic Equation: Unveiling the Ions
When NaOH and HCl react, the ions from each reactant interact. The complete ionic equation shows all the ions present in the solution before and after the reaction:
Na⁺(aq) + OH⁻(aq) + H⁺(aq) + Cl⁻(aq) → Na⁺(aq) + Cl⁻(aq) + H₂O(l)
This equation explicitly displays all the ions involved in the solution. Notice that water (H₂O) is formed as a liquid, not dissociated ions.
The Net Ionic Equation: Isolating the Key Players
The complete ionic equation includes ions that don't directly participate in the reaction. These are called spectator ions. In this case, Na⁺ and Cl⁻ are spectator ions; they appear on both the reactant and product sides of the equation, unchanged. The net ionic equation focuses only on the species that undergo a chemical change, ignoring the spectator ions.
Therefore, the net ionic equation for the reaction between NaOH and HCl is:
H⁺(aq) + OH⁻(aq) → H₂O(l)
This simplified equation accurately reflects the essence of the reaction: the combination of hydrogen ions and hydroxide ions to form water. This is the fundamental neutralization reaction between a strong acid and a strong base.
Significance of the Net Ionic Equation
The net ionic equation provides several key advantages:
-
Simplicity: It simplifies the representation of the reaction, focusing on the essential chemical transformation. This makes it easier to understand the core process.
-
Generalization: It highlights that the neutralization reaction is not unique to NaOH and HCl. Any strong acid and strong base will follow the same net ionic equation, emphasizing the generality of acid-base neutralization reactions.
-
Predictive Power: Knowing the net ionic equation allows us to predict the products of similar reactions involving different strong acids and strong bases.
-
Stoichiometric Calculations: It simplifies stoichiometric calculations, as only the reacting species are considered in mole ratios.
Beyond the Basics: Exploring Different Scenarios
While the reaction between NaOH and HCl provides a clear example, let's explore scenarios involving weak acids or bases:
Weak Acids and Strong Bases
Consider the reaction between acetic acid (CH₃COOH), a weak acid, and NaOH. Acetic acid does not fully dissociate in water. The complete ionic equation would include undissociated CH₃COOH molecules. The net ionic equation would be:
CH₃COOH(aq) + OH⁻(aq) → CH₃COO⁻(aq) + H₂O(l)
Note that the acetate ion (CH₃COO⁻) is now part of the net ionic equation because it's a product of the reaction.
Strong Acids and Weak Bases
Similarly, a reaction between HCl and ammonia (NH₃), a weak base, would have a different net ionic equation. Ammonia does not fully dissociate, so the complete and net ionic equations would reflect this:
Complete Ionic Equation: H⁺(aq) + Cl⁻(aq) + NH₃(aq) → NH₄⁺(aq) + Cl⁻(aq)
Net Ionic Equation: H⁺(aq) + NH₃(aq) → NH₄⁺(aq)
The ammonium ion (NH₄⁺) is formed as a product.
Weak Acids and Weak Bases
The reaction between a weak acid and a weak base is considerably more complex, as neither reactant fully dissociates. The equilibrium position will significantly influence the net ionic equation, and the calculation becomes more intricate, often requiring equilibrium constant (Ka and Kb) considerations.
Applications of NaOH and HCl Reactions
The neutralization reaction between NaOH and HCl has various practical applications:
-
Titrations: This reaction is fundamental in acid-base titrations, a crucial technique in analytical chemistry to determine the concentration of an unknown acid or base.
-
pH Control: Adding NaOH or HCl is a common method to adjust the pH of solutions in various industrial and laboratory settings.
-
Chemical Synthesis: This reaction is often a part of larger chemical syntheses where a specific pH is required for a reaction to proceed.
Conclusion: A Fundamental Reaction with Broad Implications
The reaction between NaOH and HCl, while seemingly simple, provides a deep understanding of fundamental chemical principles. The net ionic equation, in particular, offers a powerful tool to simplify, generalize, and predict the outcomes of acid-base reactions. By carefully considering the strengths of acids and bases involved, we can derive the appropriate complete and net ionic equations, unlocking a deeper understanding of the underlying chemistry. This fundamental reaction forms a cornerstone for various applications across chemistry and beyond, illustrating the far-reaching implications of seemingly simple chemical processes. The continued exploration of acid-base reactions and the application of net ionic equations remains crucial for advancements in various scientific fields. Understanding these concepts provides a solid foundation for more complex chemical systems and reactions.
Latest Posts
Latest Posts
-
What Percentage Is 11 Out Of 20
Mar 30, 2025
-
Cuanto Es 2 Kilos En Libras
Mar 30, 2025
-
3 Is What Percent Of 8
Mar 30, 2025
-
What Percent Of 20 Is 50
Mar 30, 2025
-
How Does A Sedimentary Rock Become An Igneous Rock
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Naoh + Hcl Net Ionic Equation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
