Number Of Valence Electrons For Potassium
Kalali
Mar 29, 2025 · 5 min read
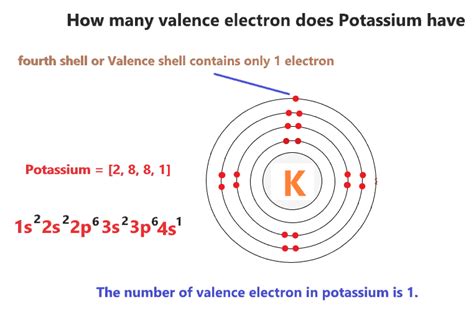
Table of Contents
Unveiling the Secrets of Potassium: A Deep Dive into its Valence Electrons
Potassium, a vital element for life, plays a crucial role in various biological processes. Understanding its electronic structure, particularly the number of valence electrons, is key to comprehending its reactivity and behavior. This comprehensive article delves into the world of potassium, exploring its atomic structure, the significance of valence electrons, and how this characteristic influences its chemical properties and biological functions.
Understanding Atomic Structure: The Foundation of Potassium's Behavior
Before diving into the valence electrons of potassium, let's establish a fundamental understanding of atomic structure. An atom consists of a nucleus containing protons and neutrons, surrounded by orbiting electrons. These electrons are arranged in specific energy levels or shells, each capable of holding a certain number of electrons. The outermost shell is known as the valence shell, and the electrons residing in this shell are called valence electrons. These valence electrons are the primary participants in chemical bonding, determining an element's reactivity.
The Electronic Configuration of Potassium (K)
Potassium, with an atomic number of 19, possesses 19 protons and 19 electrons in a neutral atom. Its electronic configuration, which describes how its electrons are distributed among the various shells, is 1s²2s²2p⁶3s²3p⁶4s¹. This notation indicates the number of electrons in each subshell. For example, 1s² means two electrons in the 1s subshell, 2s² means two electrons in the 2s subshell, and so on.
The significance of this configuration lies in the location of the outermost electron. Notice that the final electron resides in the 4s orbital. This means potassium only has one electron in its outermost shell. Therefore, potassium has one valence electron.
The Importance of Valence Electrons: The Key to Reactivity
Valence electrons play a crucial role in determining the chemical properties of an element. They are directly involved in the formation of chemical bonds with other atoms. Atoms tend to achieve a stable electron configuration, often resembling the electron configuration of a noble gas (Group 18 elements). This stable configuration is typically characterized by a full valence shell (eight electrons, except for helium with two). To achieve this stability, atoms either gain, lose, or share electrons.
Potassium's Reactivity: A Consequence of its Single Valence Electron
Because potassium possesses only one valence electron, it readily loses this electron to achieve a stable electron configuration resembling argon (1s²2s²2p⁶3s²3p⁶). This process, known as ionization, transforms potassium into a positively charged ion, K⁺. This positive charge arises because the number of protons (19) now exceeds the number of electrons (18). This ease of losing an electron is what makes potassium highly reactive, especially with elements that readily accept electrons, such as halogens (Group 17 elements).
Chemical Reactions Involving Potassium: Examples and Explanation
Potassium's reactivity is evident in its vigorous reactions with various substances.
Reaction with Water: A Vigorous Interaction
When potassium reacts with water, a highly exothermic reaction occurs. The single valence electron is transferred to a water molecule, forming a potassium ion (K⁺) and a hydroxide ion (OH⁻). The released energy is often sufficient to ignite the liberated hydrogen gas, resulting in a characteristic flame. This reaction demonstrates the strong tendency of potassium to lose its valence electron. The equation for this reaction is:
2K(s) + 2H₂O(l) → 2K⁺(aq) + 2OH⁻(aq) + H₂(g)
Reaction with Halogens: Formation of Ionic Compounds
Potassium readily reacts with halogens (fluorine, chlorine, bromine, iodine) to form ionic compounds. The single valence electron of potassium is transferred to the halogen atom, creating a potassium cation (K⁺) and a halide anion (e.g., Cl⁻, Br⁻, I⁻). These oppositely charged ions are then electrostatically attracted to each other, forming an ionic bond. For example, the reaction with chlorine produces potassium chloride (KCl), a common salt:
2K(s) + Cl₂(g) → 2KCl(s)
Biological Significance of Potassium: A Crucial Role in Life
Potassium's unique chemical properties, driven by its single valence electron, play a vital role in various biological processes. It's an essential electrolyte, crucial for maintaining fluid balance, nerve impulse transmission, and muscle contraction. Its ability to easily form ions and its involvement in electrochemical gradients are central to these functions.
Maintaining Fluid Balance: Potassium's Electrolyte Role
Potassium ions are critical in regulating fluid balance within and outside cells. The concentration gradient of potassium across cell membranes is essential for maintaining osmotic pressure and preventing cell swelling or shrinkage.
Nerve Impulse Transmission: Potassium's Contribution to Signaling
Potassium ions play a key role in the transmission of nerve impulses. The movement of potassium ions across neuronal membranes contributes to the generation and propagation of action potentials, the electrical signals that enable communication within the nervous system.
Muscle Contraction: Potassium's Essential Role in Movement
Potassium ions are crucial for muscle contraction. The concentration gradient of potassium across muscle cell membranes is necessary for the proper functioning of muscle fibers, enabling coordinated movement and bodily functions.
Applications of Potassium: Beyond Biology
The unique properties of potassium stemming from its valence electron are not limited to biological systems. It finds diverse applications in various industries.
Potassium in Fertilizers: Providing Essential Nutrients to Plants
Potassium is a vital nutrient for plant growth, and potassium-containing fertilizers are widely used in agriculture to enhance crop yields and improve plant health. Potassium aids in photosynthesis, enzyme activation, and overall plant vigor.
Potassium in Industry: Diverse Applications
Potassium compounds have various industrial applications. Potassium hydroxide (KOH) is a strong base used in soap manufacturing, and potassium carbonate (K₂CO₃) finds applications in glassmaking. Potassium permanganate (KMnO₄) is a strong oxidizing agent with disinfectant properties.
Conclusion: A Single Electron with a Profound Impact
Potassium's single valence electron has a profound impact on its chemical behavior and biological roles. Its high reactivity, its ability to easily form ions, and its involvement in crucial biological processes all stem from this fundamental characteristic. Understanding the importance of valence electrons and their influence on the properties of elements like potassium is vital for grasping their behavior in various chemical reactions and biological contexts. The information in this article underscores the interconnectedness of atomic structure, chemical reactivity, and the essential roles of elements in our world.
Latest Posts
Latest Posts
-
24 Oz Is How Many Grams
Apr 01, 2025
-
A Cuantas Pulgadas Equivale Un Metro
Apr 01, 2025
-
What Is 5 5 In Centimeters
Apr 01, 2025
-
How Many Energy Levels Does Oxygen Have
Apr 01, 2025
-
Cuanto Es 50 Grados Fahrenheit En Centigrados
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Number Of Valence Electrons For Potassium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
