How Many Energy Levels Does Oxygen Have
Kalali
Apr 01, 2025 · 5 min read
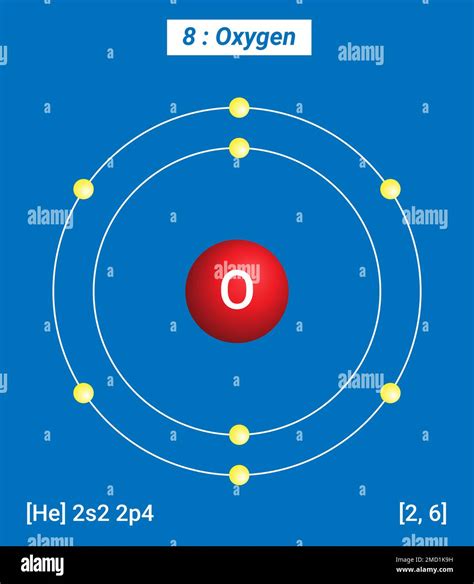
Table of Contents
How Many Energy Levels Does Oxygen Have? A Deep Dive into Electronic Structure
Oxygen, a vital element for life on Earth, possesses a fascinating electronic structure that dictates its chemical properties and reactivity. Understanding its energy levels is crucial for comprehending its role in various biological and chemical processes. This article delves into the intricacies of oxygen's electron configuration, explaining its energy levels, and exploring the implications of its electronic structure.
Understanding Electron Shells and Energy Levels
Before we dive into the specifics of oxygen, let's establish a foundational understanding of electron shells and energy levels. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons orbiting in specific energy levels or shells. These shells are not physical rings but rather regions of space where electrons are most likely to be found. Each shell can accommodate a specific number of electrons, and these electrons possess varying amounts of energy.
The shells are typically designated using numbers (1, 2, 3, etc.) or letters (K, L, M, etc.). The closer a shell is to the nucleus, the lower its energy level. Electrons in lower energy levels are more strongly bound to the nucleus than those in higher energy levels. Electrons can jump between energy levels by absorbing or releasing energy, usually in the form of photons (light). This process is fundamental to many chemical and physical phenomena.
Oxygen's Electronic Configuration: The Key to its Energy Levels
Oxygen (O) has an atomic number of 8, meaning it has 8 protons and, in its neutral state, 8 electrons. These electrons are distributed among different energy levels according to the Aufbau principle and Hund's rule. These rules dictate how electrons fill the available energy levels in an atom.
The electronic configuration of oxygen is 1s²2s²2p⁴. Let's break this down:
- 1s²: This indicates that the first energy level (n=1) contains two electrons in the s orbital. The 's' orbital is a spherical region of space.
- 2s²: The second energy level (n=2) has two electrons in the s orbital.
- 2p⁴: The second energy level also has a 'p' subshell, which can hold up to six electrons. In oxygen, this subshell contains four electrons. The 'p' subshell has three orbitals, each capable of holding two electrons.
Therefore, oxygen has three principal energy levels occupied by its electrons: n=1, n=2, and (partially filled) n=3. The n=3 energy level would become fully occupied in subsequent elements but is only partially occupied in oxygen.
It is essential to note that the energy levels aren't perfectly discrete; there's some overlap and subtle differences between the energies of the orbitals within each principal energy level (e.g. the 2s and 2p orbitals). The energies of these orbitals are influenced by several factors, including electron-electron repulsion and shielding effects.
Deeper Dive into Oxygen's Energy Levels: Orbital Interactions
The 2p subshell, with its four electrons, plays a critical role in oxygen's reactivity. According to Hund's rule, these four electrons occupy three of the 2p orbitals individually before pairing up in the same orbital. This leaves two unpaired electrons which contribute significantly to oxygen's high reactivity and ability to form two covalent bonds. This unpaired electron characteristic explains oxygen's paramagnetic nature, meaning it's attracted to a magnetic field.
The energy difference between the 2s and 2p orbitals is relatively small, leading to some orbital hybridization. While not fully hybridized like in sp³ hybridized carbon, this interaction influences the bonding angles and the overall molecular geometry of oxygen-containing compounds.
Implications of Oxygen's Electronic Structure
Oxygen's electronic structure is the basis of its crucial role in numerous processes:
-
Respiration: Oxygen's high electronegativity and the presence of two unpaired electrons allow it to readily accept electrons during cellular respiration, producing energy in the form of ATP. The energy released during electron transfer is harnessed to drive cellular processes.
-
Combustion: Oxygen's ability to readily accept electrons makes it a strong oxidizing agent, facilitating combustion reactions. These reactions release a considerable amount of energy, making oxygen essential for many industrial and energy-related applications.
-
Oxidation-Reduction Reactions: Oxygen participates in numerous redox reactions, acting as an electron acceptor (oxidizing agent). These reactions are fundamental in various chemical processes, including corrosion, photosynthesis, and the metabolism of many biological molecules.
-
Formation of Oxides: The high reactivity of oxygen leads to the formation of a vast range of oxides. These compounds play important roles in geology, materials science, and industrial processes.
Beyond the Basics: Advanced Concepts
The simplified model presented above provides a good understanding of oxygen's energy levels. However, more complex models are required for accurate predictions of oxygen's behavior in diverse situations. These include:
-
Relativistic Effects: At higher atomic numbers, relativistic effects become more pronounced and influence the energy levels of electrons. While not as significant for oxygen as for heavier elements, these relativistic effects subtly influence the properties of oxygen compounds.
-
Electron Correlation: The interactions between electrons are complex, and simple models often fail to account for electron correlation accurately. Advanced computational techniques, such as density functional theory (DFT), are used to account for these effects.
-
Excited States: When an oxygen atom absorbs energy, its electrons can transition to higher energy levels, resulting in excited states. These excited states are unstable and rapidly decay back to the ground state, often emitting light in the process. This process is crucial in understanding phenomena like aurorae and oxygen's spectral lines.
Conclusion: The Significance of Understanding Oxygen's Energy Levels
Understanding the energy levels of oxygen is not just an academic exercise. It is crucial for comprehending oxygen's unique chemical behavior, its reactivity, and its profound impact on life and the environment. From the fundamental processes of respiration to the vast array of industrial applications, oxygen's electronic structure plays a decisive role. By exploring the nuances of its electron configuration, we unlock a deeper appreciation for this essential element and its diverse contributions to the world around us. Further research continues to refine our understanding of the subtle complexities of oxygen's electronic structure, continually unveiling new insights into its remarkable properties. Advanced computational methods and experimental techniques allow us to explore increasingly detailed aspects of this fundamental element, revealing further layers of its intricate nature and enriching our comprehension of its impact on the world.
Latest Posts
Latest Posts
-
Do Nonpolar Molecules Attract Each Other
Apr 02, 2025
-
How Many Liters Is 750 Milliliters
Apr 02, 2025
-
What Is The Least Common Multiple Of 10 And 15
Apr 02, 2025
-
What Is The Boiling Point Of Seawater
Apr 02, 2025
-
What Is 8 5 As A Percentage
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about How Many Energy Levels Does Oxygen Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
