Do Nonpolar Molecules Attract Each Other
Kalali
Apr 02, 2025 · 5 min read
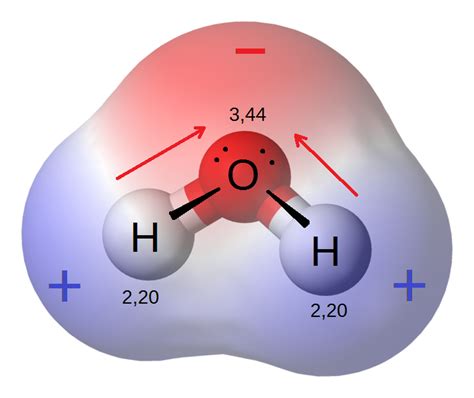
Table of Contents
- Do Nonpolar Molecules Attract Each Other
- Table of Contents
- Do Nonpolar Molecules Attract Each Other? Exploring Intermolecular Forces
- Understanding Polarity and Intermolecular Forces
- Polar Molecules: The Dipolar Dance
- Nonpolar Molecules: A Delicate Balance
- The Key Players: London Dispersion Forces (LDFs)
- The Mechanism of LDFs: Temporary Dipoles
- Factors Influencing LDF Strength
- Beyond LDFs: Other Intermolecular Forces in Nonpolar Molecules
- The Significance of LDFs: From Gases to Solids
- Examples and Applications: Seeing LDFs in Action
- Comparing LDFs to Other Intermolecular Forces
- Conclusion: The Subtle Power of Attraction
- Latest Posts
- Latest Posts
- Related Post
Do Nonpolar Molecules Attract Each Other? Exploring Intermolecular Forces
The question of whether nonpolar molecules attract each other might seem counterintuitive. After all, "nonpolar" implies a lack of permanent positive and negative poles, the very foundation of strong electrostatic attractions like those seen in ionic compounds. However, the reality is more nuanced. While nonpolar molecules don't possess permanent dipoles, they do experience attractive forces, albeit weaker than those found in polar molecules. This article will delve into the fascinating world of intermolecular forces in nonpolar molecules, explaining the mechanisms behind their attraction and the factors influencing their strength.
Understanding Polarity and Intermolecular Forces
Before diving into the attractions between nonpolar molecules, let's establish a clear understanding of polarity and the types of forces governing interactions between molecules.
Polar Molecules: The Dipolar Dance
Polar molecules possess a permanent dipole moment. This means there's an uneven distribution of electron density, resulting in a slightly positive end (δ+) and a slightly negative end (δ-). This permanent dipole allows for strong dipole-dipole interactions, where the δ+ end of one molecule is attracted to the δ- end of another. Water (H₂O) is a classic example of a polar molecule, exhibiting strong dipole-dipole interactions.
Nonpolar Molecules: A Delicate Balance
Nonpolar molecules, on the other hand, have a relatively even distribution of electron density. There's no inherent permanent dipole moment. However, this doesn't mean they are devoid of attractive forces. The electron cloud surrounding these molecules is constantly in motion. At any given instant, the electron distribution might be slightly uneven, creating a temporary, instantaneous dipole.
The Key Players: London Dispersion Forces (LDFs)
The primary force responsible for attraction between nonpolar molecules is the London Dispersion Force (LDF), also known as van der Waals forces. These forces are weak, but their cumulative effect can be significant, especially in larger molecules.
The Mechanism of LDFs: Temporary Dipoles
LDFs arise from the spontaneous fluctuations in electron distribution within a molecule. Even in a nonpolar molecule, the electrons are not perfectly static; they are constantly moving. At any given moment, a temporary, instantaneous dipole can be created where a slightly higher electron density exists in one region of the molecule. This temporary dipole then induces a dipole in a neighboring molecule, leading to an attractive interaction.
Factors Influencing LDF Strength
The strength of LDFs depends on several crucial factors:
-
Molecular Size and Shape: Larger molecules with more electrons exhibit stronger LDFs. The larger the electron cloud, the greater the probability of temporary dipole formation. Furthermore, a more elongated or less compact molecular shape tends to lead to stronger LDFs due to increased surface area for interaction.
-
Molecular Polarizability: Polarizability refers to how easily the electron cloud of a molecule can be distorted to form a temporary dipole. Molecules with easily polarizable electron clouds tend to experience stronger LDFs.
-
Number of Contact Points: The number of points where molecules can interact also contributes to the overall strength of LDFs. More contact points mean more opportunities for temporary dipoles to induce dipoles in neighboring molecules.
Beyond LDFs: Other Intermolecular Forces in Nonpolar Molecules
While LDFs are dominant in nonpolar molecules, other minor forces can also contribute to intermolecular attraction:
-
Induced Dipole-Induced Dipole Interactions: If one nonpolar molecule has a temporary dipole, it can induce a dipole in another nearby nonpolar molecule, even if that second molecule is initially perfectly symmetrical. This leads to a weak attractive force.
-
Dipole-Induced Dipole Interactions: In mixtures of polar and nonpolar molecules, the permanent dipole of the polar molecule can induce a temporary dipole in the nonpolar molecule, leading to an attractive force. This is relatively weak compared to dipole-dipole interactions between polar molecules.
The Significance of LDFs: From Gases to Solids
LDFs, despite their relatively weak nature, play a crucial role in the physical properties of many substances:
-
Boiling and Melting Points: The boiling and melting points of nonpolar substances are directly related to the strength of their LDFs. Substances with stronger LDFs tend to have higher boiling and melting points, as more energy is required to overcome the intermolecular attractions and transition to the liquid or gaseous phase. Consider the halogens: fluorine (F2) is a gas at room temperature, while iodine (I2) is a solid, reflecting the significantly stronger LDFs in the larger iodine molecule.
-
Solubility: LDFs influence the solubility of nonpolar substances. Nonpolar molecules tend to dissolve in nonpolar solvents because of the favorable interactions between their molecules. "Like dissolves like" is a useful rule of thumb in this context.
-
Surface Tension: LDFs contribute to the surface tension of liquids. The molecules at the surface experience a net inward force due to the attractions to molecules beneath them, resulting in a surface that minimizes its area.
Examples and Applications: Seeing LDFs in Action
Several examples illustrate the importance of LDFs in everyday life:
-
Noble Gases: Noble gases, such as helium, neon, and argon, are monatomic and nonpolar. Their liquefaction and solidification depend entirely on LDFs.
-
Alkanes: Alkanes, a class of saturated hydrocarbons, are nonpolar molecules. Their boiling points increase with increasing molecular weight, a direct consequence of the strengthening LDFs with increasing size.
-
Polymers: Many polymers, like polyethylene, are composed of long chains of nonpolar molecules. The extensive intermolecular interactions through LDFs contribute to their strength and physical properties.
Comparing LDFs to Other Intermolecular Forces
It's important to compare the strength of LDFs to other intermolecular forces:
-
LDFs vs. Dipole-Dipole Interactions: Dipole-dipole interactions are significantly stronger than LDFs because they involve permanent dipoles.
-
LDFs vs. Hydrogen Bonding: Hydrogen bonding, a special type of dipole-dipole interaction, is the strongest intermolecular force. It occurs when hydrogen is bonded to a highly electronegative atom like oxygen, nitrogen, or fluorine.
-
LDFs vs. Ion-Dipole Interactions: Ion-dipole interactions, involving the attraction between an ion and a polar molecule, are also stronger than LDFs.
Conclusion: The Subtle Power of Attraction
While nonpolar molecules may not exhibit the strong, permanent attractions seen in polar molecules, they still experience significant intermolecular forces. The ubiquitous London Dispersion Forces are responsible for these attractions, playing a critical role in determining the physical properties of many substances, from gases to solids. Understanding LDFs is essential for comprehending the behavior of a vast array of materials and for designing new materials with specific properties. The seemingly simple absence of a permanent dipole in nonpolar molecules belies a complex world of subtle but powerful attractive forces.
Latest Posts
Latest Posts
-
Compounds Are Represented By Chemical Formulas
Apr 05, 2025
-
Are Metals Good Insulators Of Heat
Apr 05, 2025
-
What Is 5 To The Power Of 5
Apr 05, 2025
-
150 Ml Of Water In Cups
Apr 05, 2025
-
Radius Is Half Of The Diameter
Apr 05, 2025
Related Post
Thank you for visiting our website which covers about Do Nonpolar Molecules Attract Each Other . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
