What Is Freezing In Celsius Degrees
Kalali
Mar 30, 2025 · 5 min read
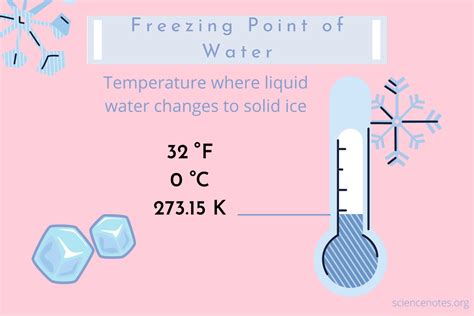
Table of Contents
What is Freezing in Celsius Degrees? A Deep Dive into the Science and Applications of 0°C
Freezing, in the context of Celsius degrees, refers to the specific temperature at which a substance transitions from a liquid state to a solid state. For water, the most common substance we associate with freezing, this transition occurs at 0° Celsius (0°C). However, understanding freezing at 0°C requires delving deeper than just this single point. This article will explore the science behind freezing at 0°C, its practical applications, and some common misconceptions.
The Science Behind Freezing at 0°C: More Than Just a Number
The freezing point of water at 0°C is a fundamental concept in science and has far-reaching implications across various fields. It's crucial to understand that this point isn't merely a random number; it's a result of the intricate interplay of intermolecular forces within the water molecule (H₂O).
Molecular Interactions and Phase Transitions
Water molecules are polar, meaning they have a slightly positive and slightly negative end due to the unequal sharing of electrons between oxygen and hydrogen atoms. This polarity leads to strong hydrogen bonds between water molecules, holding them together in a liquid state.
As the temperature drops towards 0°C, the kinetic energy of these molecules decreases. This means they move around less vigorously. At 0°C, the kinetic energy becomes insufficient to overcome the attractive forces of hydrogen bonding. The molecules become more ordered, arranging themselves into a crystalline lattice structure – the characteristic structure of ice. This phase transition is exothermic, meaning it releases heat to the surroundings.
Supercooling: When Water Refuses to Freeze
Interestingly, water can sometimes remain in a liquid state even below 0°C. This phenomenon is known as supercooling. It occurs when there are no nucleation sites – tiny imperfections or impurities – for ice crystals to form around. In the absence of these sites, the water molecules lack the necessary trigger to initiate the crystallization process. However, even a slight disturbance, such as a vibration or the addition of a small ice crystal, can initiate rapid freezing.
Factors Affecting the Freezing Point of Water
While 0°C is the standard freezing point of pure water at standard atmospheric pressure, several factors can influence this temperature:
- Pressure: Increasing pressure slightly lowers the freezing point of water. This is unusual as most substances have their freezing point increased by increased pressure. The unique behavior of water stems from the lower density of ice compared to liquid water.
- Dissolved Substances: The presence of dissolved substances (salts, sugars, etc.) lowers the freezing point of water, a phenomenon known as freezing point depression. This is why salt is used to de-ice roads in winter; it lowers the freezing point of water, preventing ice formation at temperatures slightly above 0°C.
- Impurities: The presence of impurities, even in small amounts, can affect the freezing point of water, often leading to slightly lower freezing temperatures.
Practical Applications of Freezing at 0°C
The knowledge of water's freezing point at 0°C underpins countless applications across diverse fields:
Food Preservation
Freezing at 0°C and below is a crucial technique for food preservation. Freezing halts the growth of microorganisms and slows down enzymatic reactions that can spoil food. This extends the shelf life of various food products significantly.
Cryopreservation
Cryopreservation involves freezing biological samples, such as cells, tissues, and organs, at very low temperatures to preserve them for long periods. Understanding the intricacies of freezing and the formation of ice crystals is vital to minimize damage to these sensitive materials during the freezing process.
Ice Skating and Winter Sports
The formation of ice at 0°C makes activities like ice skating and other winter sports possible. The thin layer of liquid water on the surface of ice allows for relatively low friction, enabling smooth gliding.
Water Purification
Freezing can be used as a rudimentary method of water purification. As water freezes, many impurities are excluded from the ice crystals, resulting in cleaner ice.
Construction and Infrastructure
The knowledge of water's freezing point is crucial in civil engineering and construction. Proper consideration must be given to the effects of freezing and thawing on building materials and infrastructure. This includes designing structures capable of withstanding the expansion of water as it freezes and the potential damage caused by repeated freeze-thaw cycles.
Common Misconceptions about Freezing at 0°C
Several misconceptions surround the freezing point of water:
Myth 1: Freezing always happens instantly at 0°C.
Reality: Freezing is a process, not an instantaneous event. While the transition begins at 0°C, the complete solidification of water depends on several factors, including the rate of heat removal, the presence of nucleation sites, and the volume of water.
Myth 2: All water freezes at exactly 0°C.
Reality: As discussed earlier, the freezing point of water can be influenced by various factors like pressure and dissolved substances. Pure water under standard conditions will freeze at 0°C, but any deviation from these conditions will alter the freezing point.
Myth 3: Ice at 0°C is always solid.
Reality: While ice at 0°C is predominantly solid, a thin layer of liquid water can exist on its surface due to the unique properties of water and its tendency towards supercooling.
Beyond Water: Freezing Points of Other Substances
It's important to remember that 0°C is the freezing point of water specifically. Other substances have different freezing points, some much higher and some much lower. For instance:
- Mercury: Freezes at -38.83°C
- Ethanol: Freezes at -114.1°C
- Helium: Freezes at -272.2°C (at very high pressure)
The freezing points of different substances are determined by the strength of their intermolecular forces and their molecular structure.
Conclusion: The Significance of 0°C in Our World
The freezing point of water at 0°C is a seemingly simple concept but one with profound implications across a wide range of scientific disciplines and everyday life. Understanding the science behind this phase transition, its applications, and the factors that influence it allows us to appreciate the significance of this fundamental temperature in shaping our world. From food preservation to cryopreservation, from winter sports to infrastructure design, the knowledge of 0°C plays a crucial role in numerous aspects of modern life. Further research continues to unravel the intricacies of freezing and its potential for further innovation and technological advancements.
Latest Posts
Latest Posts
-
Cuanto Es 63 Pulgadas En Pies
Apr 01, 2025
-
What Percentage Is 35 Out Of 50
Apr 01, 2025
-
How To Make A Velocity Vs Time Graph
Apr 01, 2025
-
Is The Atlantic Colder Than The Pacific
Apr 01, 2025
-
What Is The Warmest Part Of The Day
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Is Freezing In Celsius Degrees . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
